��Ŀ����
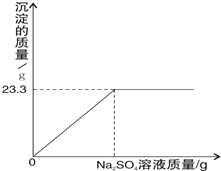
��ȡһ������BaCl2�Ĺ����ˮ���100g��Һ��Ȼ�������Һ����μ���Na2SO4��Һ������Ӧ�յ㣨ǡ����ȫ��Ӧ��ʱ���ĵ�Na2SO4��Һ����Ϊ100�ˣ���Ӧ����BaSO4�������������������Na2SO4��Һ��������ϵ��ͼ��ʾ���Իش��������⣺����ʾ��BaCl2+Na2SO4�TBaSO4��+2NaCl��
��1����ȫ��Ӧ������BaSO4����Ϊ______g��
��2����100��Na2SO4��Һ���������ʵ�����������
��3����Ӧ�յ�ʱ������Һ�����ʵ����������Ƕ��٣�����ȷ��0.1%��
��1����ȫ��Ӧ������BaSO4����Ϊ______g��
��2����100��Na2SO4��Һ���������ʵ�����������
��3����Ӧ�յ�ʱ������Һ�����ʵ����������Ƕ��٣�����ȷ��0.1%��

��1����ͼ�п����Կ�������23.3g�����������������ӣ�����ȫ��Ӧ������BaSO4����������Ϊ23.3g��
�ʴ�Ϊ23.3g��
��ǡ����ȫ��Ӧʱ����Na2SO4������Ϊx����Ӧ���ɵ�NaCl������Ϊy
BaCl2+Na2SO4�TBaSO4��+2NaCl
142233 117
x23.3g y
=
��
=
x=14.2g y=11.7g
��2��Na2SO4��Һ���������ʵ�����������
��100%=14.2%
��3����Ӧ�յ�ʱ����NaCl ��Һ���ʵ���������=
��100%��6.6%
��100��Na2SO4��Һ���������ʵ���������Ϊ14.2%����Ӧ�յ�ʱ������Һ�����ʵ�����������6.6%��
�ʴ�Ϊ23.3g��
��ǡ����ȫ��Ӧʱ����Na2SO4������Ϊx����Ӧ���ɵ�NaCl������Ϊy
BaCl2+Na2SO4�TBaSO4��+2NaCl
142233 117
x23.3g y
| 233 |
| 142 |
| 23��3g |
| x |
| 233 |
| 117 |
| 23.3g |
| y |
x=14.2g y=11.7g
��2��Na2SO4��Һ���������ʵ�����������
| 14.2g |
| 100g |
��3����Ӧ�յ�ʱ����NaCl ��Һ���ʵ���������=
| 11.7g |
| 100g+100g-23.3g |
��100��Na2SO4��Һ���������ʵ���������Ϊ14.2%����Ӧ�յ�ʱ������Һ�����ʵ�����������6.6%��

��ϰ��ϵ�д�
�����Ŀ
