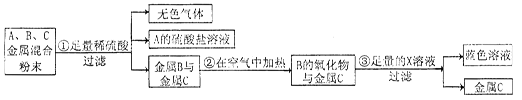��Ŀ����
��������������벻�����ϣ���ÿ��1�֣���10�֣�
��1������ϰ���ϰѽ���������ͭ�������ֽ���ͳ��Ϊ����𡱣��ڡ����˳���аѽ����� ����λ��������һλ�����÷���������ǿ�Ļ��˳��ij�������뵽ϡ��������Һ��Ϊdz��ɫ����Ӧ�Ļ�ѧ����ʽΪ ��
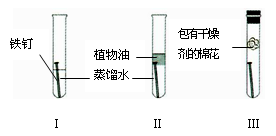
��2��Ϊ�˷�ֹˮ��ͷ���⣬����泣��һ�����������ԭ���ǣ� ������ʱ�����е����Ż���ù��Ǹ�����ԭ����______________________________________��
��3��CO����ұ��ҵԭ�ϣ��������彡���к���д����¯������CO��ԭ�������Ļ�ѧ��Ӧ����ʽ�� ��ú���ж�����ΪCO��ѪҺ�� ��ϣ��������ȱ����
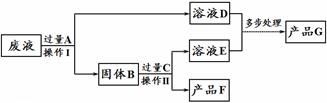
��4������һ��ͭ��п��Ϸ�ĩ���ڿ����г�����պ���ϡ����������ǡ���ܽ⣬�ټ���һ���������ۣ���ַ�Ӧ����ˣ��õ���������Һ����������һ���� ����Һ������һ���� ��
��5�����ÿյ�����ͭ��Ȧ����������ͭ���������� �ԣ�Ҫ��֤þ������ͭ�Ļ��˳����ѡ���˴�ĥ������˿������ͭ��Һ������Ϊ������Ҫ����Һ��______ ______��Һ��
��1��ͭ��Fe+H2SO4==FeSO4+H2�� ��2������ˮ�Ϳ���������������3��Fe2O3+3CO 2Fe+3CO2 ��Ѫ�쵰�ף�4��ͭ������п���������� (5) ���硢����þ
2Fe+3CO2 ��Ѫ�쵰�ף�4��ͭ������п���������� (5) ���硢����þ
����

��7�֣�ʹ���ܶ�С��ǿ�ȴ��þ�Ͻ��ܼ����������أ��Ӷ������������ĺͷ����ŷš�
|
��3����þ��ԭ��MgO�ɴӺ�ˮ�л�á�С������þ�����ᷴӦ��ķ�Һ��ģ��Ӻ�ˮ�л�ȡMgO�Ĺ��̣�ʵ�����£�
����1����������Һ�У��߽���߷�������CaO����MgCl2��ȫ����Ϊֹ�����˵�Mg(OH)2���塣������8.4g CaO��
����2����Mg(OH)2������ȷֽ�ΪMgO��ˮ������MgO������Ϊ4.0g ��
�ٲ���2��Mg(OH)2�ֽ�Ļ�ѧ����ʽΪ ��
��ͨ������MgO����������������Һ�к�MgCl2������m= g��
�۷���ʵ�����ݣ���֪������з����Ļ�ѧ��Ӧ�У�
CaO+H2O==Ca(OH)2��Ca(OH)2+MgCl2==CaCl2+Mg(OH)2���� ��
��2�֣���ͬ�����ʣ�����ɡ��ṹ���������ʵȷ��棬�������������������ԡ�������һ�㣬���������Ǹ��õ�ѧ�û�ѧ���������
| �� �� | ������ | ������ |
| Fe��Cu��Mg | ���ܺ�AgNO3��Һ��Ӧ | |
| HCl��H2SO4��HNO3 | | HCl�в�����Ԫ�� |
| C��CO��H2 | | CO�ǻ���������ǵ��� |
| CuSO4��MgSO4��ZnSO4 | ��������ˮ | |