��Ŀ����
����Ŀ��ijƷ��άC����Ƭ��ǩ��ͼ1��ʾ��Ϊ�о������ʲ��ⶨ����NaHCO3�������������̽�����̡�

��1��άC����Ƭ�������л�����г�ζ���ϡ������ᡢ_______��
��2����άC����ƬͶ��ˮ�У��۲쵽�д������ݲ�������Ҫ����Ϊ�����ᣨH3C6H5O7����NaHCO3������Ӧ�������Ƹ÷�Ӧ��
3NaHCO3+H3C6H5O7= Na3C6H5O7+3 H2O+_______��
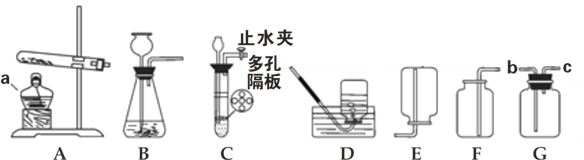
��3��Ϊ�ⶨNaHCO3���������ͼ2װ�ã������������������

ʵ�鲽�裺��ȡ��Ʒm gװ������У���С�Թ��з���С�ʹ��������ӣ��ټ�������ϡ���ᣬ�ٰѽ��ҷŵ��Թ��У���500 mLϴƿ��װ��һ�����ı���̼��������Һ����С�Թ�С�ķ���ϴƿ����ͼ2�����Ǻ�ϴƿ�ĸ��ӣ����ڴ����������ϣ������ܽ⣬����Ƭ���������ã���Ӧ�ӽ���ɣ���������ֱ��ϴƿ����Һ������������Ͳ��ȡϴƿ��������Һ�����ΪV mL��ʹ�ý��ҵ�Ŀ����_______�������ӵ�������_______������̼�����ƴ���ˮ�����Է�ֹ_______����֪���¶���CO2�ܶ����� g/mL����άC����Ƭ��NaHCO3������_______��
��4�����в������ܵ��²ⶨ���ƫС����_______��
a��С�Թ������������ϴƿ�� b��ʵ�������������Ͳ����
c������m gάC����Ƭʱ��������������ȷ��������ƽָ��ƫ��������
d��άC����Ƭ��װ�������ʱ��������
��5��ijͬѧ���װ�ã���ͼ3���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ�����_______��
���ⶨNaHCO3������Ҫ�����������_______��_______��
a����Ʒ����m g
b����Ӧǰ��Aװ������a g��a1 g
c����Ӧǰ��Bװ������b g��b1 g
d����Ӧǰ��Cװ������c g��c1 g
��ѡ������һ�����ݣ���ʾNaHCO3����_______��ָ����װ�õ�һ��ȱ����_______��

���𰸡� ���Ǻ�ά����C 3CO2 ��ֹάC����Ƭ��NaHCO3�������ᷴӦ����CO2�ݳ� ʹ��Ӧ��� CO2�ܽ� 21��v/11m bd ��Y����������б��ʹ����������Ʒһ�� ab �������10��ac ![]() ��
��![]() ���ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ����
���ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ����
����������������Ϣ֪����1��άC����Ƭ�������л�����г�ζ���ϡ������ᡢ���Ǻ�ά����C����2����άC����ƬͶ��ˮ�У��۲쵽�д������ݲ�������Ҫ����Ϊ�����ᣨH3C6H5O7����NaHCO3������Ӧ���÷�Ӧ��3NaHCO3+H3C6H5O7= Na3C6H5O7+3H2O+ 3CO2 ������3���ⶨNaHCO3������ʹ�ý��ҵ�Ŀ����. ��ֹάC����Ƭ��NaHCO3�������ᷴӦ����CO2�ݳ��������ӵ�������ʹ��Ӧ��֡�����̼�����ƴ���ˮ�����Է�ֹCO2�ܽ⡣��֪���¶���CO2�ܶ����� g/mL����άC����Ƭ��NaHCO3�����ǡ����ɶ�����̼�����Ǧ�vg. NaHCO3��CO2,, ![]() ��
��![]() ,x��21��v/11g. ��4���������ܵ��²ⶨ���ƫС����b��ʵ�������������Ͳ����. d��άC����Ƭ��װ�������ʱ��������. ��5���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ����ǽ�Y����������б��ʹ����������Ʒһ�ˡ����ⶨNaHCO3������Ҫ�����������a����Ʒ����m g��b����Ӧǰ��Aװ������a g��a1 g��a����Ʒ����m g��c����Ӧǰ��Bװ������b g��b1 g����ѡ������һ�����ݣ���ʾNaHCO3������
,x��21��v/11g. ��4���������ܵ��²ⶨ���ƫС����b��ʵ�������������Ͳ����. d��άC����Ƭ��װ�������ʱ��������. ��5���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ����ǽ�Y����������б��ʹ����������Ʒһ�ˡ����ⶨNaHCO3������Ҫ�����������a����Ʒ����m g��b����Ӧǰ��Aװ������a g��a1 g��a����Ʒ����m g��c����Ӧǰ��Bװ������b g��b1 g����ѡ������һ�����ݣ���ʾNaHCO3������![]() ��
��![]() �����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
�����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
��

����Ŀ����һ�������£�һ���ܱ������ڷ���ij��Ӧ����÷�Ӧǰ������ʵ��������±���ʾ��
����˵������ȷ����
���� | a | b | c | d |
��Ӧǰ������/g | 30 | 20 | 10 | 15 |
��Ӧ�������/g | x | y | 0 | 10 |
A. �μӷ�Ӧ��c��d����֮����2:1 B. x��ȡֵ��Χ�ǣ�0��x��30
C. ��y��20ʱ���÷�Ӧ�ǻ��Ϸ�Ӧ D. x+y=65