��Ŀ����
Ϊ�˲ⶨijʯ��ʯ��Ʒ��̼��Ƶĺ�����ȡ10.0gʯ��ʯ��Ʒ�������ȫ�������ձ��У�������������ϡ���ᣬ�ձ�����ʢ���ʵ�������Ϊ80.0g����Ӧ�����в���ձ�����ʢ���ʵ���������Ӧʱ���¼���±���| ��Ӧʱ��/�� | 2 | 4 | 6 | 8 | 10 | |
| �ձ�����ʢ��������/�� | 80.0 | 79.0 | 78.3 | 77.9 | 77.8 | 77.8 |
��1����Ӧ�������ų�������̼������Ϊ______��
��2����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
���𰸡����������ȸ��������غ㶨��������ɵĶ�����̼�������������������ļ��٣�Ȼ��Ѷ�����̼���������뻯ѧ����ʽ����Ϳ�������뷴Ӧ��̼��Ƶ�������
����⣺��1�����������غ㶨�ɿɵã����ɵĶ�����̼�������ǣ�80g-77.8g=2.2g
��2����10gʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
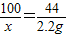
x=5g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ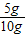 ×100%=50%
×100%=50%
�𣺣�1�����ų�������̼2.2g
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ50%
�����������ѶȲ��Ǻܴ���Ҫ�����������غ㶨�ɵ�Ӧ�ú��ݻ�ѧ����ʽ�����йصļ��㣬����ѧ����������������ͽ�������������
����⣺��1�����������غ㶨�ɿɵã����ɵĶ�����̼�������ǣ�80g-77.8g=2.2g
��2����10gʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
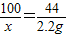
x=5g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ
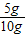 ×100%=50%
×100%=50%�𣺣�1�����ų�������̼2.2g
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ50%
�����������ѶȲ��Ǻܴ���Ҫ�����������غ㶨�ɵ�Ӧ�ú��ݻ�ѧ����ʽ�����йصļ��㣬����ѧ����������������ͽ�������������

��ϰ��ϵ�д�
�����Ŀ
ʯ��ʯ�����е�һ����Ҫ�����Դ��Ϊ�˲ⶨijʯ��ʯ��Ʒ��̼��Ƶĺ�����ijͬѧ�ø�ʯ��ʯ��Ʒ��ϡ���ᷴӦ�����ʼȲ�����ˮҲ�������ᷴӦ��������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ���
��1��������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��2�����ϱ������з���������ȡʯ��ʯ������ϡ�����������Ϊ ʱ������ʯ��ʯ�е�̼�����ϡ�����е��Ȼ���ǡ����ȫ��Ӧ��
| ��һ�� | �ڶ��� | ������ | |
| ��ȡʯ��ʯ�Ͻ������/g | 25 | 25 | 50 |
| ����ϡ���������/g | 120 | 160 | 100 |
| ������̼������/g | 9.9 | 9.9 | 9.9 |
��2�����ϱ������з���������ȡʯ��ʯ������ϡ�����������Ϊ