��Ŀ����
����Ŀ����ѧ������������������ء�
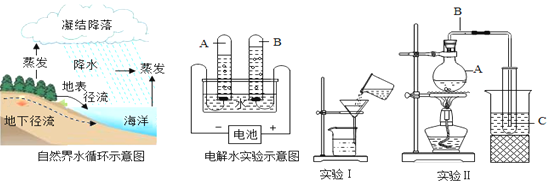
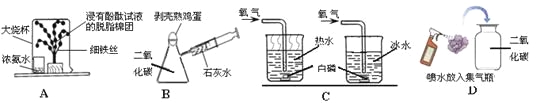
��1����ѧ�����������ߣ��������������ѡ����Ӧ�������ա�
A���� B�ƾ� C����̿ D��ȼ��
��ʵ���ҳ��õ�ȼ����_________;
����������ʳƷ����____________
�ۿ����������ζ������__________;
��2017��5�£��ҹ��ɹ��Ӻ����ɵ�����Դ��______________��
��2��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���______��ʯ�ͺ���Ȼ��������ʹ�û�ʯȼ�ϻ����һЩ�������⣬����������Ҫ��������Դ����֪����һ������Դ��_______���й����ʵ����2017��5��18���������ҹ��ɹ���ɿ�ȼ����ʵ�鿪�ɹ�������ȼ��������ɷ���Ҫ�Ǽ��飬��ȼ�յĻ�ѧ��Ӧ����ʽΪ___________________��
��3�����з��𡢷����İ�ȫ֪ʶ��˵����ȷ���� ___________(����ĸ����)��
A�ڼ���վ���ܽ����Ͳ����ֻ�
Bҹ�䷢�ֳ�����ú��й©���������Ƽ��
C¥�����֣�����ʱ��ʪë����ס�ڱ�,���¿������棬Ѹ���뿪�����ֳ�
D��ۼӹ�������֯����ú�����Ҫ�Ͻ��̻�
���𰸡�B A C D ú ̫���� CH4+2O2![]() CO2+2H2O ACD
CO2+2H2O ACD
��������
��1���پƾ����п�ȼ�ԣ���ʵ���ҳ�����ȼ�ϣ�
�ڵ�����ѧ�����ȶ�������ʳƷ��װ�������ı������ǵ�����
�ۻ���̿���������ԣ������������������ζ��
��2017��5�£��ҹ��ɹ��Ӻ����ɵ�����Դ�ǿ�ȼ����
��2�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ�����������ڿ���ʹ�õ�����Դ�����ܡ�̫���ܡ������ܡ����ܵȣ�����ȼ�����ɶ�����̼��ˮ����Ӧ����ʽΪ��CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
��3��A���ڼ���վ���ܽ����Ͳ����ֻ�����������ȼ��ȼ������������ȷ��
B��ҹ�䷢�ֳ�����ú��й©�����ܿ��Ƽ�飬��������ȼ��ȼ���������ʴ���
C��¥�����֣�����ʱ��ʪë����ס�ڱǣ����¿������棬Ѹ���뿪�����ֳ�������ȷ��
D����ۼӹ�������֯����ú�����Ҫ�Ͻ��̻𣬹���ȷ��

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д�����Ŀ��С껶Լ��е�������������Ũ�����Ȥ������������ȡ�����ռ���һ�����壬����ʵ���Ҷ�������о���
[��������]��������Ļ�ѧ�������ƣ�������ȼ������Ҫ������¶�Ϊ40��C������ȼ�յ����ֱ���ʽΪ____________________��
[�������]С�̽�������������Ƿ�Ϊ������������
[����ʵ��]ȡһֻ250mL�ļ���ƿ������ˮ���ռ�һƿ�������壬Ȼ���һС��ȼ�ŵ�������뼯��ƿ�У��۲쵽����ȼ�յĸ���������С�����Ϊ���������Ǵ�����������ʯ��ȼ�յ����ֱ���ʽΪ____________________��
[ʵ�鷴˼]��ʦָ������ʵ�鲢����֤��С�������������һ���Ǵ����������� ��ʵ�鲻��֤�����ռ�����Ϊ����������ԭ����____________________��
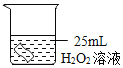
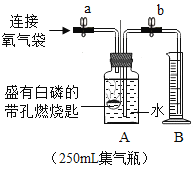
[����̽��]Ϊ�ⶨС����������������ĺ�����ͬѧ������ʦ��ָ�����������ͼ��ʾװ�á�����ʵ�飬���ظ���Ρ�Ϊȷ������ɹ��ڼ���ҩƷ��Ӧ���еIJ���Ϊ_____________________��Ȼ���ֹˮ��a��b����A�л�������һ��������ر�ֹˮ��a��b��A��ˮ���뵽B�У�B��ˮ�����Ϊ200ml (��ѹǿ������������Բ���) �������۹���ȼ����,������Ϩ����ȴ�����£���ֹˮ��b��ʵ��������¼�������£�
ʵ����� | 1 | 2 | 3 | 4 | 5 |
B��ʣ�����/ml | 120 | 40 | 39 | 37 | 36 |
[���ݴ���]��֪�����ϱ������У���1������ƫ��ϴ��������������ݴ���ʱӦɾȥ��С�������������������������Ϊ__________%��
[��������]
���µ�1�����ݲ����ϴ����Ŀ���ԭ����____________________��
����Ŀ�����������������������й㷺Ӧ�á�ʵ��С��Թ��������ijЩ���ʽ����о���
�������ȶ���
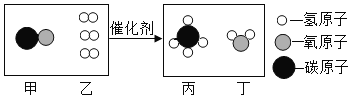
��1����ͼ��ʾ����ʵ�飬��������ֽ�Ļ�ѧ����ʽΪ_____
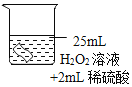
��2��������ˮ���ռ�O2��ԭ����_____������3.2gO2ʱ�ֽ�Ĺ������������Ϊ_____g��
��3��̽���¶ȶԹ�������ֽ����ʵ�Ӱ��ͬѧ�ǽ��������µ�ʵ�飬ʵ���������±���
ʵ����� | �� | �� | �� |
H2O2��Һ��Ũ��/% | 30 | 30 | 30 |
H2O2��Һ�����/mL | 6 | 6 | 6 |
�¶�/�� | 20 | 35 | 55 |
MnO2������/g | 0 | 0 | 0 |
�ռ�O2�����/mL | 0 | 1.9 | 7.8 |
��Ӧʱ�� | 40min | 40min | 40min |
�ɴ˵ó��Ľ�����_____��
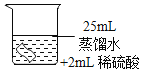
������ʴ��
���������ϣ�H2O2��Һ�и�ʴ�ԡ�
������ʵ�飩��ͭƬ�ֱ������3����Һ�н���ʵ�飬���±���
��� | �� | �� | �� |
ʵ�� |
|
|
|
һ��ʱ�������� | �����Ա仯 | ͭƬ��С����Һ��������������ϸС���� | �����Ա仯 |
����������ۣ���4��ʵ��ٵ�������_____��
��5��ͭƬ����ʴ�ķ�Ӧ���£���ȫ�÷�Ӧ�Ļ�ѧ����ʽ��Cu+H2O2+H2SO4=CuSO4+_____��
����˼��������6��ijͬѧ�����ʵ����У���������5���ķ�Ӧ�⡣��������һ����Ӧ������ϸС���ݲ������÷�Ӧ�ķ�Ӧ��Ϊ_____��