��Ŀ����
����Ŀ��������ˮ�����������������Ҫ��Դ��
��1���ӽ��ӵ�ˮԴȡ����ˮ�����á�������____�Ȳ�����ȥˮ�в��������ʣ�Ȼ��������̿�����û���̿��____�ԣ���ȥ��ζ��
��2������______����������ˮ��Ӳˮ������ˮ�������г���____�ķ�������ˮ��Ӳ�ȡ�
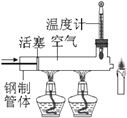
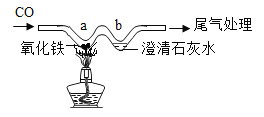
��3����ͼ��ˮ���ʵ��װ��ͼ����ͼ���жϲ����������Թ���____���1����2������
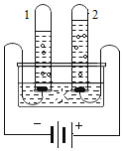
��4���ҹ�������һ�����ʹ�����ʵ�����ڹ����·ֽ�ˮ����Ӧ�Ļ�ѧ����ʽΪ__��
���𰸡����� ���� ����ˮ ��� 1 2H2O 2H2 ��+ O2��
2H2 ��+ O2��
��������
��1�������ܰ�ˮ�������з������ȥ���������ʣ����ԣ�ȡ�������������á����������˵Ȳ�����ȥˮ�в��������ʣ���ˮ����ˮ�����û���̿�������ԣ��ɳ�ȥˮ������ζ���ʣ�������ˣ�������
��2��Ӳˮ�к��н϶�Ŀ����Ը�þ����������ˮ��ϻ���������ĸ�������ˮ�в������������Ŀ����Ը�þ����������ˮ��ϻ������������ĭ���ʿ���ʹ�÷���ˮ����Ӳˮ����ˮ��Ӳˮ�еĿ����Ը�þ������������ת��Ϊ�����Ը�þ���������ʹ�ü�����еķ�������ˮ��Ӳ�ȣ��������ˮ����С�
��3�����ˮʱ���������⣬�����һ�������Բ������������Թ�1�����1��
��2���������֪��ˮ�ڴ������յ�������������������������Ӧ�ķ���ʽΪ��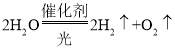 �����
�����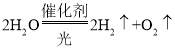 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��������ˮ����500 mL������ƿ�м���2�װ��ǡ�1.5 gС�մ�ע������ˮ���ټ���1.5 g�����ᣨC6H8O7������������ƿ�ǣ�ҡ�ȡ�
�����ϣ���Ӧԭ����3NaHCO3 + C6H8O7=C6H5O7Na3 + 3H2O+ 3CO2��
���A��B��������ѡ1������������������𣬰�A�Ʒ֡�
A | B |
��1����������̼����Ԫ�ص�������Ϊ_____�� ��2���Ƶõ���ˮ�������ζ���������ݲ�������ζ��������_____�� | ��1����������̼Ԫ�ص���������Ϊ37.5%�������ʽΪ_____�� ��2���������������������ƿ�ǵ�ԭ����_____�� |