��Ŀ����
��6�֣�ij��ѧ��ȤС��������ϵ�֪�����γ�NaCl�⣬������MgCl2��CaCl2�Լ���ɳ�����ʡ�Ϊ�˽������ᴿ����������²������̣�
�������������Ϣ�ش��������⣺
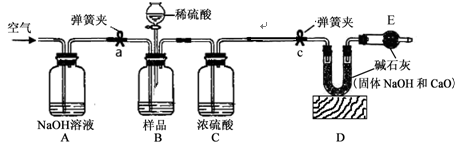
��1�������������Ϊ_______________��
��2�������������������Һ��������ѧ��Ӧ�ķ���ʽΪ ��
��3���������̼������Һ��ʵ��Ŀ���� ��
����������������Һ�к��е��������� ��
����
��4��ijͬѧ���þ��α�����ͬѧ����Ҫ�࣬ԭ�������__________��AB
_______ ____
| A����ֽ������Һ���Ǿ����� |
| B������ʱδ�ò���������һЩҺ�彦�� |
| C���ᴿ�����þ�����δ��ȫ���� |
| D���ܽ�ʱˮ����̫�٣�ʹʳ��δ��ȫ�ܽ� |
(1)���� ��1�֣�����2��2NaOH + MgCl2 = 2NaCl + Mg(OH)2����2�֣���
(3) ��ȥ��Һ�еĸ����ӣ�����Ȼ��ơ�����1�֣�Cl- ��OH- ��CO32-��1�֣�����4��A��C��2�֣�.
����

 ��У����ϵ�д�
��У����ϵ�д�ȼú�����������к��ж�������ֱ���ŷŻ���Ⱦ����������ˮ��Ӧ�γ�������Ⱦ������ij����С�����ú�ˮ��ȥ���������乤�����̼���ͼ��
��ش��������⣺
��1��������������Ļ��ϼ��� ��
��2������ʯ���к�ϡ����Ļ�ѧ����ʽ�� ��
��3�������ᣨH2SO3������������������Ϊ���ᣬд����Ӧ�Ļ�ѧ����ʽ ��
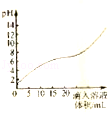
��4��Ϊ���о������ʣ����������ѳ�ȥ�Ķ����������ռ�ܶ����������İٷֱȡ������¶ȡ�������SO2Ũ�ȵĹ�ϵ�����ǽ�����̽��ʵ�顣ʵ�������£�
| ʵ����� | �¶�/�� | ������SO2Ũ��/10-2g��L-1 | ������/% |
| I | 23 | 2.5% | 99.5 |
| II | 23 | 3.2% | 97.1 |
| III | 40 | 2.5% | 94.3 |
��5��Ŀǰ����ѧ�������о���һ�������½���ϩ��C2H4����������ϳ�ȥ���������䷴Ӧ�Ĺ��̿ɷ�Ϊ����������
��һ����O2��Cu+��Ӧ����Cu+(O2)
�ڶ�����Cu+(O2)��SO2��Ӧ����Cu+(SO3)2
��������Cu+(SO3)2��C2H4��Ӧ����S��CO2��H2O��Cu+��
�ٷ�Ӧ��Cu+�������� ��
�ڳ�ȥ����������ܷ�Ӧ�Ļ�ѧ����ʽ�� ��
������л�ѧ���������³��������ʣ�
| A����ʯ�� | B������ | C����ʯ�� | D������̿ |
��1�������������˵�θҺ�У��ܰ�����������______��
��2������ʳƷ���������_______��
��3�������ڷ�����ߵ���_______��
��4������������������ ��

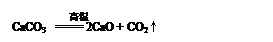 ��
��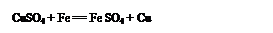 ��
��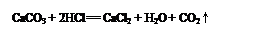 ��
��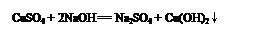 ��
��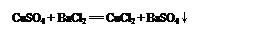 ��
��