��Ŀ����
����Ŀ�����۵ĽǶ���ʶ���ʼ���仯�������ͼʾ�ش��������⡣
��1���Ӻ���Ͽ���ͼ1��ʾ�仯����_________�仯������������������ѧ���������۽ǶȽ���ͼ1��ͼ2��ʾ�������仯�ı���������______________________________��
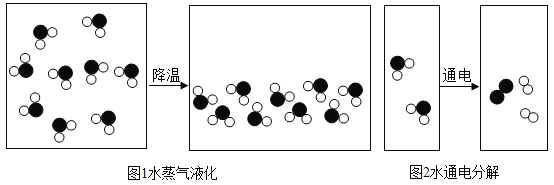
��2��д������ͼ2��ʾ�仯�Ļ�ѧ����ʽ________________________���÷�Ӧ�Ļ�����Ӧ������_______��
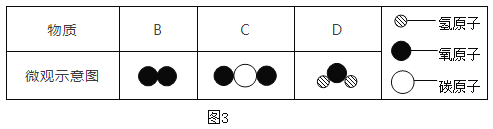
��3��ͼ3�У�B��C��D���������������������______________(�ѧʽ)����һ��������A��6.4g��Bǡ����ȫ��Ӧ��������4.4gC��3.6gD�������A��Ԫ����__________��
���𰸡����� ͼ1�з�������û�б䣬���Ӽ�ļ�϶�����ı䣬��ͼ2�з���������˸ı䣬����������� 2H2O![]() 2H2��+O2�� �ֽⷴӦ CO2��H2O ̼Ԫ�غ���Ԫ��
2H2��+O2�� �ֽⷴӦ CO2��H2O ̼Ԫ�غ���Ԫ��
��������
��1���Ӻ���Ͽ���ͼ1��û�����������ɣ��������������仯�������ı仯��֪ͼ1��ͼ2�ı��������ǣ�����ˮ���ӱ���û�иı䣬ֻ�Ƿ��Ӽ����С�������������仯����ˮ���ӱ�Ϊ����Ӻ������ӣ������˻�ѧ�仯��
��2����ͼʾ��֪��ͼ2��ʾ�ķ�Ӧ�ǵ��ˮ��������������������ѧ����ʽΪ��2H2O![]() 2H2��+O2�����÷�Ӧ���С�һ��ࡱ���ص㣬������Ӧ����Ϊ�ֽⷴӦ��
2H2��+O2�����÷�Ӧ���С�һ��ࡱ���ص㣬������Ӧ����Ϊ�ֽⷴӦ��
��3���������Ĺ��ɿ�֪��B��C��D�ֱ���O2��CO2��H2O���������������CO2��H2O��
���������֪����һ��������A��6.4g��Bǡ����ȫ��Ӧ��������4.4gC��3.6gD��4.4gC������Ԫ�ص�����Ϊ��4.4g��![]() ��100%=3.2g��̼Ԫ�ص�����Ϊ4.4g-3.2g=1.2g��3.6gD������Ԫ�ص�����Ϊ��3.6g��
��100%=3.2g��̼Ԫ�ص�����Ϊ4.4g-3.2g=1.2g��3.6gD������Ԫ�ص�����Ϊ��3.6g��![]() ��100%=3.2g������Ԫ��3.6g-3.2g=0.4g����ΪBΪ��������Ϊ6.4�ˣ�����C��D����Ԫ�ص�����3.2g+3.2g=6.4g����A�в�������Ԫ�أ�A��ֻ����̼Ԫ�ء���Ԫ�ء�
��100%=3.2g������Ԫ��3.6g-3.2g=0.4g����ΪBΪ��������Ϊ6.4�ˣ�����C��D����Ԫ�ص�����3.2g+3.2g=6.4g����A�в�������Ԫ�أ�A��ֻ����̼Ԫ�ء���Ԫ�ء�
�ʴ�Ϊ��
��1�������仯��ͼ1�з�������û�б䣬���Ӽ�ļ�϶�����ı䣬��ͼ2�з���������˸ı䣬����������ӡ���2��2H2O![]() 2H2��+O2�����ֽⷴӦ����3��CO2��H2O��̼Ԫ�غ���Ԫ�ء�
2H2��+O2�����ֽⷴӦ����3��CO2��H2O��̼Ԫ�غ���Ԫ�ء�