��Ŀ����
����Ŀ��ij�о�С����������ᾧ����Ʒ�ֽ���ﲢ�ⶨ�������������������ʲ����뷴Ӧ�������ᾧ�壨H2C2O42H2O�����������ʼ�����
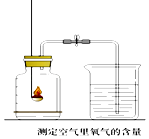
��1�����ȷֽ���ᾧ�������˵�װ��������ͼ1��ĸ��ţ��� 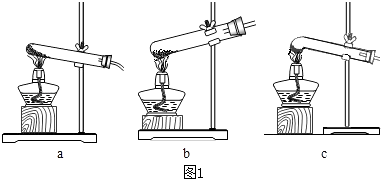
��2��ͼ2����֤�ȷֽ�����к�CO��CO2��װ�� ������a��b�����Ʒֱ�����
��֤������CO2�������� �� ֤������CO�������� �� D�з�Ӧ�Ļ�ѧ����ʽ�� ��
��װ��A�������� �� ���ҵ������� �� 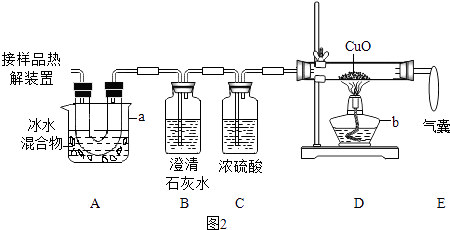
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�����������������������·�����
�۵� | �е� | ���ȶ��� | ��Ӧ |
101��C��102��C | 150��C��160��C���� | 100.1��Cʧȥ�ᾧˮ��175��C�ֽ��CO2 �� CO��H2O | ��Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
�ٳ�һ������Ʒ����ͼװ�ý���ʵ�飬���װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ��������أ���ԭ������У�COδ��ȫ��Ӧ�� ��
�ڳ�ȡ8.75g���ᾧ����Ʒ����50.00g��Һ��ȡ10.00g��Һ��������ϡ���ᣬȻ��μ�25.00g3.16%KMnO4��Һ��ǡ�÷�Ӧ��ȫ��
����֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O����KMnO4��Һ��ɫ��25.00g3.16%KMnO4��Һ��KMnO4������g���������Ʒ�е�����������[д��������̣�M2��H2C2O4��=90��M2��H2C2O42H2O��=126��M2��KMnO4��=158]��
���𰸡�
��1��c
��2���ձ����ƾ��ƣ�B�г����ʯ��ˮ����ǣ�D�к�ɫ�����ɺ�ɫ��CO+CuO ![]() Cu+CO2����ȥ������������ֹ�Զ�����̼�ļ���������ţ��ռ�һ����̼����ֹ��Ⱦ����
Cu+CO2����ȥ������������ֹ�Զ�����̼�ļ���������ţ��ռ�һ����̼����ֹ��Ⱦ����
��3�����ɵ�ͭ�ֱ��������Ϻ죻0.79
���������⣺��1��������۵�ϵͣ����������ۻ�����cװ�ü��Ȳ���ʱ�����������������������Ȳ��ᣮ ���c����2��������a��b�����Ʒֱ����ձ����ƾ��ƣ�
����ձ����ƾ��ƣ���֤������CO2�������ǣ�B�г����ʯ��ˮ����ǣ�֤������CO�������ǣ�D�к�ɫ�����ɺ�ɫ��
���B�г����ʯ��ˮ����ǣ�D�к�ɫ�����ɺ�ɫ��
D������ͭ��һ����̼�ڼ���ʱ��Ӧ������ͭ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ�ǣ�CO+CuO ![]() Cu+CO2 ��
Cu+CO2 ��
���CO+CuO ![]() Cu+CO2 �� ��װ��A�������ǣ���ȥ������������ֹ�Զ�����̼�ļ���������ţ����ҵ������ǣ��ռ�һ����̼����ֹ��Ⱦ������
Cu+CO2 �� ��װ��A�������ǣ���ȥ������������ֹ�Զ�����̼�ļ���������ţ����ҵ������ǣ��ռ�һ����̼����ֹ��Ⱦ������
�����ȥ������������ֹ�Զ�����̼�ļ���������ţ��ռ�һ����̼����ֹ��Ⱦ��������3����һ����̼���ַ�Ӧ�����ɵ�ͭ���±����������ض��ܹ����¼������ʵ������ʵ��ֵƫ�ͣ�
������ɵ�ͭ�ֱ��������ڸ��������Һ����ɫ�Ϻ�ɫ�ģ�
����Ϻ죮
25.00g3.16%KMnO4��Һ��KMnO4������Ϊ��25.00g��3.16%=0.79g��
���0.79��
��10.00g��Һ�к����ᾧ�������ΪX��
��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��֪��
5H2C2O42H2O��5H2C2O4�� | 2KMnO4 �� |
630 | 316 |
x | 0.79g |
![]() =
= ![]()
X=1.575g��
50.00g��Һ�к����ᾧ�������Ϊ��1.575g��5=7.875g��
���ᾧ�����������Ϊ�� ![]() ��100%=90%��
��100%=90%��
����Ʒ�в��ᾧ�����������Ϊ90%��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
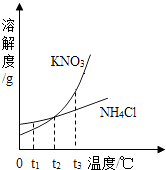
�ƸԿ�����ҵ��ϵ�д�����Ŀ�������ĸ�ͼ���У�����ȷ��ʾ�Է�Ӧ�仯��ϵ���ǣ� ��
|
|
|
|
A����һ����ϡ�����м�ˮϡ�� | B��һ���¶��£������������Һ�м�������� | C������һ�����ĸ�����ع��� | D����������Ȼ�þ�Ļ����Һ�м�����������Һ |
A.A
B.B
C.C
D.D