��Ŀ����
����Ŀ������ͬѧ��ʵ���ҷ�����һƿ���ڷ��õĹ���������Һ������Ϊ����Һ�����ʵ�����������С�ˣ���Ҫ���²ⶨ�������Լ�ƿ�ı�ǩ������������ʵ�飺��������Һ34.0 g�����ձ�����Ȼ�������м���������������(0.5 g)��ֱ�����ٷų���������ٳ����ձ��л������������������Ϊ33.7 g�����㣺
(1)������������Ԫ�ص���������(����һλС��)____________��
(2)�����ƿ��Һ�����ʵ���������_________________��
���𰸡� 94.1% 5%
�����������⿼���˸��ݻ�ѧ����ʽ�ļ��㡣���������������غ����������������Ȼ�����÷���ʽ�еı�����ϵ�����Ҫ�������ʵ�������
��1��������������Ԫ�ص�����������![]() ��100%=94.1%��
��100%=94.1%��
��2���������̵�������0.5g�������ɵ����������Ϊ��34.0g+0.5g-33.7g=0.8g��
��������0.8g������Ҫ�������������Ϊx��
2H2O2![]() 2H2O+O2��
2H2O+O2��
68 32
x 0.8g
![]() x=1.7g
x=1.7g
��Һ�����ʵ���������=![]() ��100%=5%��
��100%=5%��

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�����Ŀ��ijͬѧ�Ѹ������������غͶ������̵Ļ����31.5gװ����Թ��У�������ȡ���������ڲ�ͬʱ�̲ⶨ�Թ���ʣ��������ʵ�����(���±�)��
��Ӧʱ�䣯min | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
ʣ���������/g | 28.9 | 26.3 | 23.7 | 21.9 | 21.9 |
�����������ݣ�����������⣺
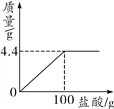
(1)��ȫ��Ӧ����������������Ϊ___________g��
(2)��ʣ��������������ˮ�ܽ���˵õ���Һ55g(��������������ˮ)��������Һ�����ʵ����������Ƕ���?(��ȷ��0.1��) ___________
����Ŀ���ҹ�������ѧ�Һ�°����������ˡ������Ƽ������ԭ������Ҫ��һ������ʳ��ˮ���Ⱥ�ͨ������NH3��CO2�Ʊ�NaHCO3����ѧ����ʽ��NaCl+ NH3+CO2+H2O= NaHCO3��+NH4Cl
ij��ȤС����ʵ����ģ��ù��̣����Ͼ���IJ���ش��������⣺
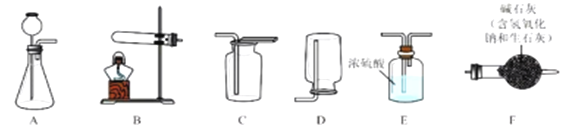
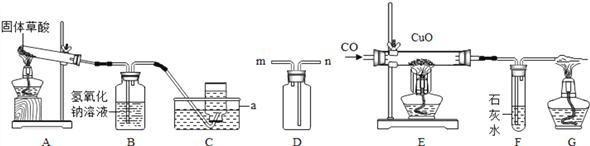
I�������Ʊ�
��1��������̼�����Ʊ�
ʵ���ҳ�����ʯ��ʯ��ϡ���ᷴӦ�Ʊ�CO2���仯ѧ����ʽΪ_____________��Ӧѡ��������ռ�װ��Ϊ______(ѡ��װ�ö�Ӧ����ĸ)��
��2�������Ʊ�
ʵ���ҳ����������հ�������ֹ��Ⱦ������ʵ�����Ʊ�NH3�ķ�Ӧԭ��Ϊ��Ca(OH)2(��)+2NH4Cl(��) ![]() CaCl2+2H2O+2NH3������Ҫ�Ʊ�������NH3����ѡװ�õ���ȷ����˳��Ϊ_____��______(ѡ��װ�ö�Ӧ����ĸ)��ʵ�����Ʊ�O2Ҳ���������Ʊ�NH3�ķ���װ�ã�д���ø÷���װ���Ʊ�O2�Ļ�ѧ����ʽ__________��
CaCl2+2H2O+2NH3������Ҫ�Ʊ�������NH3����ѡװ�õ���ȷ����˳��Ϊ_____��______(ѡ��װ�ö�Ӧ����ĸ)��ʵ�����Ʊ�O2Ҳ���������Ʊ�NH3�ķ���װ�ã�д���ø÷���װ���Ʊ�O2�Ļ�ѧ����ʽ__________��
II��NaHCO3�Ʊ�
���� | NaHCO3 | NH4Cl |
�ܽ��/g(20��) | 9.6 | 37.2 |
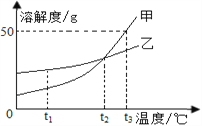
��3�����ݱ����е��ܽ�����ݣ�����20��������NaHCO3�ܹ��ȴ���Һ�нᾧ������ԭ��________��
��4���ù�������һ����NH4Cl��ũҵ�����г�������______________��