��Ŀ����
��ͨ��ͭ����ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е�����Ϊ�ⶨ��ͭ��ͭ������������ȡ��Ʒ10g�����Ĵ������м���ϡ����ʹ֮��ַ�Ӧ��ʵ�����ݼ�¼���±���
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 8.7 | 7.4 | 7 | 7 |
��1���ù����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2����ͭ��ͭ����������Ϊ______��
��3�����������ϡ�������������������
�⣺��1����Ϊп����ϡ���ᷴӦ��������п����������ͭ����ϡ���ᷴӦ��
�ʷ�Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2����
�ʴ�Ϊ��Zn+H2SO4=ZnSO4+H2����
��2��ʣ���������Ϊ7g�����ټ��٣�����Ʒ��ͭ������Ϊ7g����ͭ��ͭ����������=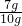 ��100%=70%��
��100%=70%��
�ʴ�Ϊ��70%��
��3������Ʒ��п��ȫ��Ӧʱ���μӷ�Ӧ��ϡ�������ʵ�����Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 98
10g-8.7g x
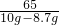 =
=
��ã�x=1.96g��
��ϡ������Һ�����ʵ���������=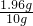 ��100%=19.6%��
��100%=19.6%��
���������ϡ�����������������19.6%��
��������1������п����ϡ���ᷴӦ��ͭ����ϡ���ᷴӦ���ʸ��ݷ�Ӧ��������T��д����ѧ����ʽ��
��2������п����ϡ���ᷴӦ��ͭ����ϡ���ᷴӦ����˵�ϡ�������ʱ��ʣ�����ȫ��Ϊͭ������ʵ���¼���ݿ�֪�����Ĵ��������ϡ���Ტδ������������ʱʣ�����Ϊͭ������ͭ����Ʒ���������ɼ����ͭ��ͭ������������
��3�����ڵ�һ��ʵ����ϡ������ȫ��Ӧ����˿���ʵ�������е�һ����п��ϡ�����������ϵ���ж���Ʒ��п��ȫ��Ӧʱ����ϡ�������ʵ�����������ϡ�������ʵ���������Һ�������������ϡ������Һ�����ʵ�����������
���������⿼���˸��ݻ�ѧ����ʽ������������������ļ��㣻ͨ�����ݶ������Ĵ�ʵ��ʣ��Ĺ�����ͭ����һ��ʵ��ϡ������ȫ��Ӧ�ǽ����Ĺؼ���
�ʷ�Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2����
�ʴ�Ϊ��Zn+H2SO4=ZnSO4+H2����
��2��ʣ���������Ϊ7g�����ټ��٣�����Ʒ��ͭ������Ϊ7g����ͭ��ͭ����������=
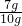 ��100%=70%��
��100%=70%���ʴ�Ϊ��70%��
��3������Ʒ��п��ȫ��Ӧʱ���μӷ�Ӧ��ϡ�������ʵ�����Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 98
10g-8.7g x
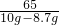 =
=
��ã�x=1.96g��
��ϡ������Һ�����ʵ���������=
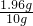 ��100%=19.6%��
��100%=19.6%�����������ϡ�����������������19.6%��
��������1������п����ϡ���ᷴӦ��ͭ����ϡ���ᷴӦ���ʸ��ݷ�Ӧ��������T��д����ѧ����ʽ��
��2������п����ϡ���ᷴӦ��ͭ����ϡ���ᷴӦ����˵�ϡ�������ʱ��ʣ�����ȫ��Ϊͭ������ʵ���¼���ݿ�֪�����Ĵ��������ϡ���Ტδ������������ʱʣ�����Ϊͭ������ͭ����Ʒ���������ɼ����ͭ��ͭ������������
��3�����ڵ�һ��ʵ����ϡ������ȫ��Ӧ����˿���ʵ�������е�һ����п��ϡ�����������ϵ���ж���Ʒ��п��ȫ��Ӧʱ����ϡ�������ʵ�����������ϡ�������ʵ���������Һ�������������ϡ������Һ�����ʵ�����������
���������⿼���˸��ݻ�ѧ����ʽ������������������ļ��㣻ͨ�����ݶ������Ĵ�ʵ��ʣ��Ĺ�����ͭ����һ��ʵ��ϡ������ȫ��Ӧ�ǽ����Ĺؼ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ͨ��ͭ����ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е�����Ϊ�ⶨ��ͭ��ͭ������������ȡ��Ʒ10g�����Ĵ������м���ϡ����ʹ֮��ַ�Ӧ��ʵ�����ݼ�¼���±���
����������ݣ��ش��������⣺
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 8.7 | 7.4 | 7 | 7 |
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
��ͨ��ͭ����ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е�����Ϊ�ⶨ��ͭ��ͭ������������ȡ��Ʒ10g�����Ĵ������м���ϡ����ʹ֮��ַ�Ӧ��ʵ�����ݼ�¼���±���
����������ݣ��ش��������⣺
��1���ù����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ͭ��ͭ����������Ϊ ��
��3�����������ϡ�������������������
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 8.7 | 7.4 | 7 | 7 |
��1���ù����з�����Ӧ�Ļ�ѧ����ʽΪ
��2����ͭ��ͭ����������Ϊ
��3�����������ϡ�������������������
��ͨ��ͭ����ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е�����Ϊ�ⶨ��ͭ��ͭ������������ȡ��Ʒ10g�����Ĵ������м���ϡ����ʹ֮��ַ�Ӧ��ʵ�����ݼ�¼���±���
����������ݣ��ش��������⣺
��1���ù����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2����ͭ��ͭ����������Ϊ______��
��3�����������ϡ�������������������
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 8.7 | 7.4 | 7 | 7 |
��1���ù����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2����ͭ��ͭ����������Ϊ______��
��3�����������ϡ�������������������