��Ŀ����
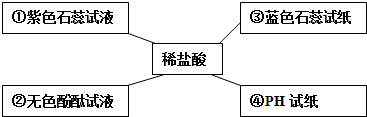
��11�֣�����֪ʶ������һ����Ҫ��ѧϰ������ͼΪijУ��ѧѧϰС���С��ͬѧ���Ƶ����Ļ�ѧ��������ʾ��ͼ��������ش��������⣺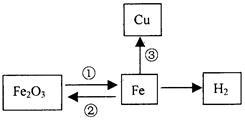

(1)��Ӧ���п��� �� (�ѧʽ)����ԭ����
��д����¯�з����ķ�Ӧ�ٵĻ�ѧ����
ʽ�� �� ��
д����Ӧ�۵Ļ�ѧ����ʽ �� ��
�Ƿ�Ӧ�ڵ���ÿ���д����ĸ�����ʴ��Ϊ��ֹ�÷�Ӧ�ķ��������dz������ڸ�������Ϳˢ�����������������ȷ����������ַ����Ĺ�ͬԭ������ֹ����
�� �� �Ӵ���
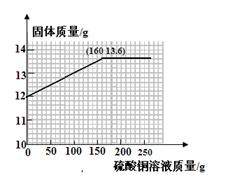
�ȸû�ѧѧϰС��Ϊ�˷������������ĺ���������������ʵ���о���ȡ12g������10%�����ᷴӦ���������˼�������������ų����������Ĺ�ϵͼ��������ͼ������˵�������������ʲ�����ˮ���������ᡢ����ͭ��Һ��Ӧ����
������ȫ��Ӧ��ȥ���������Ϊ �� g��
��������������������Ϊ���٣���д��������̣����������С�����һλ��
��
��5��ijͬѧ�г������¼���ʽ��
����������պ÷�Ӧ��ȫʱ������Һ����= (146+12-a)g
С���Ըü���ʽ��������ɣ���ָ�����еĴ��� ��
��
��6��С���������һ��ʵ�鷽������12g��������20%������ͭ��Һ��Ӧ���������������ĺ��������㻭����������ͭ��Һ��������������仯��ϵ�����ߡ�
(1)C��H2��CO��0.5�֣�
��2�� ����
Fe2O3+3CO====2Fe+3CO2
Fe+CuSO4==FeSO4+Cu����Fe+CuCl2==FeCl2+Cu�� Fe+Cu��NO3��2==Fe��NO3��2+Cu ��
(3)������ˮ����������ˮ��
��4����146��0.5�֣�
�ڣ���4��
�⣺�������ᷴӦ�����ĵ�����Ϊx�� 0.5��
 Fe + 2HCl �� FeCl2 + H2��(1��)
Fe + 2HCl �� FeCl2 + H2��(1��)
56 73
x 146 g��10%="14.6" g
x =" 11.2g" 0.5��
������������������Ϊ=��11.2g��12g����100% = 93.3% 1��
��������������������Ϊ93.3%��
��5����Һ��������������������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�����
��6��ץס��㡢�۵㼰�������Ƹ��֣�����Ҫ��ע��Щ�㣬ץס��㡢�۵㼰�����������֣���һ�������֣��������ξ�Ϊֱ�ߣ�ƫ��̫���֣�����㡢�۵㼰��������û�д��������£����ò���λ����ֻ����ǰ�벿�֣�����ȷ���Ŀ��ʵ��۷֡���2�֣�����:
��

��2�� ����
Fe2O3+3CO====2Fe+3CO2
Fe+CuSO4==FeSO4+Cu����Fe+CuCl2==FeCl2+Cu�� Fe+Cu��NO3��2==Fe��NO3��2+Cu ��
(3)������ˮ����������ˮ��
��4����146��0.5�֣�
�ڣ���4��
�⣺�������ᷴӦ�����ĵ�����Ϊx�� 0.5��
 Fe + 2HCl �� FeCl2 + H2��(1��)
Fe + 2HCl �� FeCl2 + H2��(1��)56 73
x 146 g��10%="14.6" g
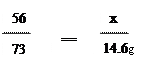 | |||
|
x =" 11.2g" 0.5��
������������������Ϊ=��11.2g��12g����100% = 93.3% 1��
��������������������Ϊ93.3%��
��5����Һ��������������������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�����
��6��ץס��㡢�۵㼰�������Ƹ��֣�����Ҫ��ע��Щ�㣬ץס��㡢�۵㼰�����������֣���һ�������֣��������ξ�Ϊֱ�ߣ�ƫ��̫���֣�����㡢�۵㼰��������û�д��������£����ò���λ����ֻ����ǰ�벿�֣�����ȷ���Ŀ��ʵ��۷֡���2�֣�����:
��

��ϰ��ϵ�д�
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
�����Ŀ
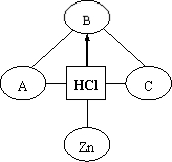 33������֪ʶ������һ����Ҫ��ѧϰ��������ͼ�ǹ������ữѧ���ʵ�֪ʶ���磬��--����ʾ���������������ܷ�����Ӧ����������ʾһ������ת������һ�����ʣ�A��C���ڲ�ͬ���Ļ�����������֪ʶ���磮
33������֪ʶ������һ����Ҫ��ѧϰ��������ͼ�ǹ������ữѧ���ʵ�֪ʶ���磬��--����ʾ���������������ܷ�����Ӧ����������ʾһ������ת������һ�����ʣ�A��C���ڲ�ͬ���Ļ�����������֪ʶ���磮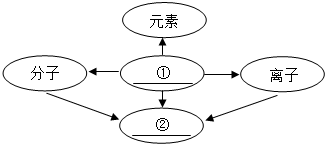 ����֪ʶ������һ����Ҫ��ѧϰ������С��ͬѧ�����ʵ���ɺͽṹ�����֪ʶ�����˹��ɣ�������д�հף����������ƣ�
����֪ʶ������һ����Ҫ��ѧϰ������С��ͬѧ�����ʵ���ɺͽṹ�����֪ʶ�����˹��ɣ�������д�հף����������ƣ�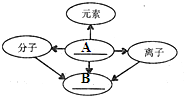 18������֪ʶ������һ����Ҫ��ѧϰ������С��ͬѧ��������ɺͽṹ�����֪ʶ�����˹��ɣ�������д�հף����������ƣ�
18������֪ʶ������һ����Ҫ��ѧϰ������С��ͬѧ��������ɺͽṹ�����֪ʶ�����˹��ɣ�������д�հף����������ƣ�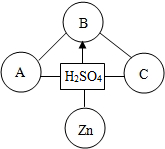 ��2013?��³ľ�룩����֪ʶ������һ����Ҫ��ѧϰ��������ͼ�ǹ������ữѧ���ʵ�֪ʶ���磺��-����ʾ���������������ܷ�����Ӧ����������ʾһ��������ת��Ϊ��һ�����ʣ�A��B��C�ֱ����ڲ�ͬ���Ļ������A��B��C�����ǣ�������
��2013?��³ľ�룩����֪ʶ������һ����Ҫ��ѧϰ��������ͼ�ǹ������ữѧ���ʵ�֪ʶ���磺��-����ʾ���������������ܷ�����Ӧ����������ʾһ��������ת��Ϊ��һ�����ʣ�A��B��C�ֱ����ڲ�ͬ���Ļ������A��B��C�����ǣ�������