��Ŀ����
Сǿ�Ե��ص�ʯ��ʯ��ʯ��Ʒ���м�⣬ȡ��100g����ʯ��ʯ��Ʒ����300gϡ�����3�μ��룬ʵ������������ݼ��±�����֪ʯ��ʯ��Ʒ�к������ʲ�����ˮ���������ᷴӦ��������㣺
��1���±���m����ֵӦ��Ϊ ��
��2����Ʒ��̼��Ƶ����ʵ����� mol��
��3���μӷ�Ӧ��������������� �����ݻ�ѧ����ʽ��ʽ���㣩������ȷ0��1%��
��1���±���m����ֵӦ��Ϊ ��
��2����Ʒ��̼��Ƶ����ʵ����� mol��
| ��� | ����ϡ����������g�� | ʣ�����������g�� |
| ��1�� | 100 | 60 |
| ��2�� | 100 | m |
| ��3�� | 100 | 12 |
��3���μӷ�Ӧ��������������� �����ݻ�ѧ����ʽ��ʽ���㣩������ȷ0��1%��
��1��20 ��2��0��88 ��3��29��2%
�����������1���ɵ�1�ι���������С40g����2�ι�������Ҳ��С40g,����m=60g-40g=20g��
��2����Ʒ��̼��Ƶ����ʵ�����=
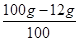 =0��88mol
=0��88mol��3��������ΪX mol
CaCO3 + 2HCl = CO2��+ H2O +CaCl2 ��1�֣�
1 2
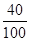 =0��4mol Xmol
=0��4mol Xmol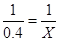
X=0��8mol
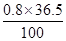 ��100%=29��2%
��100%=29��2%
��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ