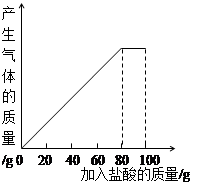��Ŀ����
Ϊ�ⶨij��ʯ��ʯ��Ʒ��̼��Ƶ�������������������ʵ�飻��װ��5.0gʯ��ʯ��Ʒ���ձ��У�����23.6gϡ���ᣬǡ����ȫ��Ӧ���ձ���ʣ�����ʵ���������ʱ��仯��ϵ���±�����ʯ��ʯ�е����ʲ��μӷ�Ӧ��������ˮ�����ɵ�����ȫ���ݳ���
��
��1���������ɶ�����̼���ٿˣ�
��2����Ʒ��̼��Ƶ�����������
��3������Ӧ���ձ��е�ʣ�����ʼ���30.5��ˮ��ɲ�������Һ����й��ˣ����Եõ���Һ���������������Ƕ��٣�
| ʱ��/min | t1 | t2 | t3 | t4 | t5 | t6 |
| �ձ���ʣ�����ʵ�������/g | 28.35 | 28.1 | 27.85 | 27.6 | 27.5 | 27.5 |
��1���������ɶ�����̼���ٿˣ�
��2����Ʒ��̼��Ƶ�����������
��3������Ӧ���ձ��е�ʣ�����ʼ���30.5��ˮ��ɲ�������Һ����й��ˣ����Եõ���Һ���������������Ƕ��٣�
��1��1.1g�� ��2��50%�� ��3��5%
�����������1������ʯ��ʯ��Ʒ�е�̼�������ϡ���ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���ݳ������ʷ�Ӧ���ձ���ʣ�����ʵ����������С�����������غ㶨�ɿ�֪�����ٵ�������Ϊ���ɵĶ�����̼�����������ʵ�����ݿ�֪���ձ�������ʣ�����ʵ���������27.5g���������ɵĶ�����̼������5g+23.6g-27.5g=1.1g��
��2�����ݶ�����̼����������Ϸ�Ӧ�Ļ�ѧ����ʽ���ɼ������Ʒ��̼��Ƶ������������������Ʒ��̼��Ƶ�����������
�⣬����Ʒ��CaCO3������Ϊx��
CaCO3��2HCl��CaCl2��H2O��CO2��
100 44
x 1.1g
100:44=x��1.1g
��ã�x=2.5g
����Ʒ��̼��Ƶ���������=
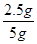 ��100%��50%
��100%��50%����Ʒ��̼��Ƶ���������Ϊ50%��
��3���������⣬����ʯ��ʯ��Ʒ�е�̼�������ϡ���ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���ݳ�������ʯ��ʯ�е����ʲ��μӷ�Ӧ��������ˮ���ʵ�����23.6gϡ���ᣬ����ǡ����ȫ��Ӧʱ��������Һǡ��Ϊ��Ӧ���ɵ�CaCl2��Һ����ͬ�����ݻ�ѧ����ʽ����������ɵ��Ȼ����������ٳ������������Һ�����������������Һ����������������
�⣬�跴Ӧ���ɵ�CaCl2������Ϊy��
CaCO3��2HCl��CaCl2��H2O��CO2��
111 44
y 1.1g
111:44=y��1.1g
��ã�y=2.775g
��õ���Һ��������������=
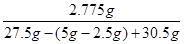 ��100%��5%
��100%��5%�𣺵õ���Һ����������������5%��
�����������ǹ��ڻ�ѧ����ʽ�ļ����⣬��Ҫ������ͼ������Ӧ����ʽ�������ͽ����ѧ�����е��й����⣬Ҫ��ѧ���н�ǿ�����ݷ�������������Ĺؼ���Ҫ��ͨ�����ݵıȽϣ���������غ㶨�ɣ��жϳ���Ӧ���ɵĶ�����̼��������Ȼ�������صĻ�ѧ��Ӧ����ʽ����������֪����δ֪��Ӧ�������������㼴�ɣ�ע�����Ҫ�淶��

��ϰ��ϵ�д�
�����Ŀ