��Ŀ����
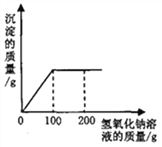
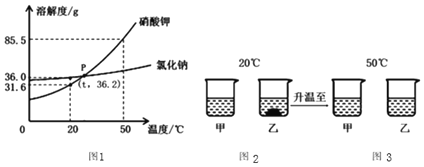
����Ŀ����һ�ձ���ʢ��22.3��̼���ƺ��Ȼ�����ɵĹ������������ˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��
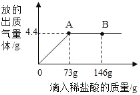
��1�����μ���73��ϡ����ʱ���ų������������Ϊ_____�ˣ�
��2�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��������ǣ�д��ѧʽ��_____��
��3�����μ���73��ϡ����ʱ����A�㣩���ձ���Ϊ��������Һ����ͨ����������������ʵ�����������_____��
���𰸡�4.4 NaCl��HCl 23.4��
��������
��ų������������Ϊz��
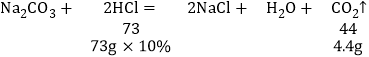
![]()
��֮�ã�z=4.4g
(2)��ͼʾ����֪�������μ�ϡ������ͼ��A��ʱ,������ȫ��Ӧ���ٵμ�ϡ������ͼ��B��ʱ���������ʣ�ࡣ����Һ�е�����ΪNaCl��HCl��
(3)�⣺�跴Ӧ������NaCl������Ϊx���������Na2CO3������Ϊy��
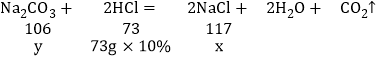
![]()
��֮�ã�y=10.6g x=11.7g
��Ӧ������NaCl������=11.7g+22.3g-10.6g=23.4g��
���(1)4.4��(2)NaCl��HCl��(3)������Һ�����ʵ�����Ϊ23.4g��

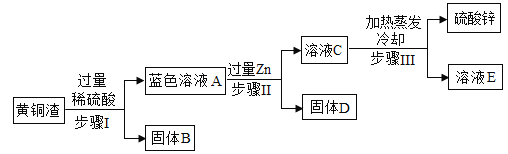
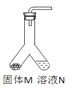
����Ŀ�����е�װ����ȡ����(��ȡʱ��װ���еĹ�����Һ����)���±�Ϊ�������ȡ��װ�úͷ�Ӧ�Ļ�ѧ����ʽ
��ȡ�����װ�� | ����M | ��ҺN | ��ȡ������ | ��Ӧ�Ļ�ѧ����ʽ |
| ����ʯ | _______�� | CO2 | __________�� |
п�� | ϡ���� | ________�� | __________�� |