��Ŀ����
ij��ѧ��ȤС���ͬѧ��һ��пͭ�Ͻ�ӹ��ɷ�ĩ������ȡ��ͬ��������Ʒ���ݣ���ϡ�������ͼ��ʾ���ʵ�װ�ý���ʵ�飬���Բⶨ�Ͻ���ͭ��п������������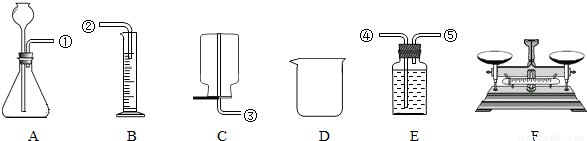
ʵ�鷽�����£�
��1����ͬѧ�ķ�����ȡ������Ʒһ�ݣ�����ϡ���������岻�ٲ�������������������������������ٸ��ݻ�ѧ����ʽ���м��㣮���ø÷�������ʵ�飬Ӧѡ�õ�װ���У�����ĸ��ʾ��______����װװ��ʱ���ܿڵ�����˳���ǣ���ܽӿڴ���ţ�______��
��2����ͬѧ�ķ�����ȡ������Ʒһ�ݣ�����������ϡ�����ַ�Ӧ���Ƴ�ͭ���������ٽ��м��㣮���ø÷�������ʵ�飬��Ҫ���ӵIJ���������______��
��3����ͬѧ��Ϊֻѡ��D��F����ʵ�飬Ҳ���Դ��ԵIJⶨ���Ͻ���п��ͭ�������������������ͬѧ��ʵ�鷽�������ø÷�������ʵ�飬���Ҫ�����Ĵ������٣�Ӧ�ó�����������______��
���𰸡���������1������Ҫ������ȡ������װ�ã��ռ�������װ�ã�����Ҫ��ӵIJ��������������װ�ã����ӵ�˳��ؼ����ڢܢ�������
��2��������֪����Ҫ����һ������װ�ã�
��3��������֪����ͬѧ�ķ�����ͨ����Ӧǰ���������������������������Գ��������ݾ��Ƿ�Ӧǰ�������ձ�����Һ������������Ӧ���ձ�����Һ��ʣ���������������
����𣺣�1��������֪��ѡ���װ������ȡ������װ�ã��ռ�������װ�ã�����Ҫ��ӵIJ��������������װ�ã�װ�����ӵ�˳���Dz���������ٴӢݽ��룬�������Ӳ���ѹ����ˮ�ų����Ӣڽ�����Ͳ�����ɲ��ˮ�������Ҳ����������������ʴ�Ϊ��A��E��B���٢ݢܢ�
��2��������֪����Ҫ����һ������װ�ã�������Ҫ�������У�����̨��©�����ձ������������ʴ�Ϊ��©����������
��3����ͬѧ�ķ�����ͨ����Ӧǰ���������IJ��������������������Ҫ���������ݾ��Ƿ�Ӧǰ�������ձ�����Һ������������Ӧ���ձ�����Һ��ʣ����������������ʴ�Ϊ����Ӧǰ�������ձ�����Һ������������Ӧ���ձ�����Һ��ʣ�������������
������������ڿ��鳣��������ѡ�䣬�Լ���ȷ�����ӣ�Ҫ��ѧ����ƽʱ��ʵ�������������������Լ����֡����Ե�������
��2��������֪����Ҫ����һ������װ�ã�
��3��������֪����ͬѧ�ķ�����ͨ����Ӧǰ���������������������������Գ��������ݾ��Ƿ�Ӧǰ�������ձ�����Һ������������Ӧ���ձ�����Һ��ʣ���������������
����𣺣�1��������֪��ѡ���װ������ȡ������װ�ã��ռ�������װ�ã�����Ҫ��ӵIJ��������������װ�ã�װ�����ӵ�˳���Dz���������ٴӢݽ��룬�������Ӳ���ѹ����ˮ�ų����Ӣڽ�����Ͳ�����ɲ��ˮ�������Ҳ����������������ʴ�Ϊ��A��E��B���٢ݢܢ�
��2��������֪����Ҫ����һ������װ�ã�������Ҫ�������У�����̨��©�����ձ������������ʴ�Ϊ��©����������
��3����ͬѧ�ķ�����ͨ����Ӧǰ���������IJ��������������������Ҫ���������ݾ��Ƿ�Ӧǰ�������ձ�����Һ������������Ӧ���ձ�����Һ��ʣ����������������ʴ�Ϊ����Ӧǰ�������ձ�����Һ������������Ӧ���ձ�����Һ��ʣ�������������
������������ڿ��鳣��������ѡ�䣬�Լ���ȷ�����ӣ�Ҫ��ѧ����ƽʱ��ʵ�������������������Լ����֡����Ե�������

��ϰ��ϵ�д�
�����Ŀ
 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����


 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������