��Ŀ����
����Ŀ��ȫ����ÿ�걻��ʴ��ĵĸ�������Լռȫ�����������ʮ��֮һ�������Ƕ����ĸ�ʴ������������ɵ�̽����
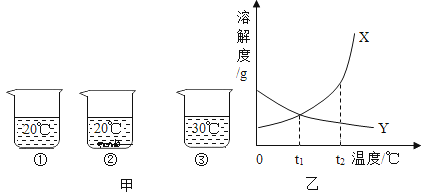
����һ��̽�������������
�α�ʵ���У�����������Ҫʱ��ϳ���ijʵ��С��������¸Ľ�ʵ�飮

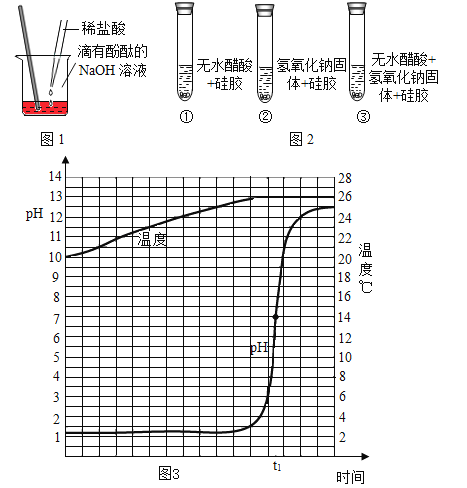
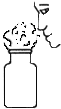
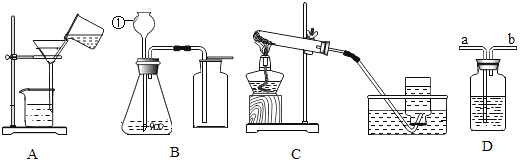
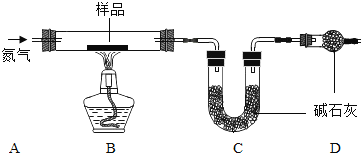
��1�����װ�õ������ԣ����Ӻ�װ�ã��رշ�Һ©���Ļ�������C���ܽ��뵽װ��ˮ���ձ��У��ȼ�װ�ã�˵�����������õ�������______��
��2����Ӧ��ʼ��װ���з�Ӧ������______��MnO2������______��
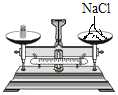
��3��4���Ӻ�۲죺A����˿��Ȼ������B����˿����Ұ���D����˿��Ȼ��������ʵ��˵����������Ҫ��______��______�йء�B��D��ʵ������Ա�˵�������������������һ����Ҫ������______��
��������ⶨ��������
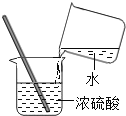
����ijɷ���ҪΪFe2O3H2O��������������FeCO3��ij��ȤС�������ͼװ�òⶨ����������ɣ�ȡһ�߶ȸ�ʴ�ĸ�������������������������������Ϊ20.08g����N2��Χ�У���ּ��ȵ��������ټ��٣��ù����н����Ƿ�����![]() ��
��![]() ���Ҳ���Ҫ����װ���п����Ա�ʵ���Ӱ�죮����ʯ�ҳɷ֣�CaO��NaOH����������ʵ���Է���������Fe2O3H2O178��FeCO3116��
���Ҳ���Ҫ����װ���п����Ա�ʵ���Ӱ�죮����ʯ�ҳɷ֣�CaO��NaOH����������ʵ���Է���������Fe2O3H2O178��FeCO3116��
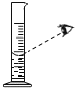
��1������Ӧǰ��Ƶ�Cװ���������ֱ�Ϊ100.00g��102.24g����Ӧ���ɵ�CO2��ˮ������������Ϊ______g��Dװ���м�ʯ�ҵ�������______��
��2����������ʵ�����ݣ���������ɣ�����B��C֮������һ��װ�ü��ɣ���װ�����ƺ�ҩƷΪ______��______��
��3�������Ľ����m��H2O��=1.80g��m��CO2��=0.44g�����������������ɣ�
����������Fe2O3H2O������=______g��
����������FeCO3������=______g��
�����������������������=______��
��4����20.08g������������������ϡ���ᣬ��ַ�Ӧ�������������Ƿ���H2��______������������������������ͨ������˵��_____������������H2SO4��Fe2(SO4)3�������Һ���ȷ����ķ�ӦΪ��Fe+Fe2(SO4)3=3FeSO4��Fe2(SO4)3����Է�������Ϊ400��
���𰸡�C���ܿ������� ������������ ���� ˮ ���� ������Ũ�� 2.24 ��ֹ�����еĶ�����̼��ˮ����װ��C�� ϴ��ƿ Ũ���� 17.8 1.16 5.6% �� �⣺�����������ᷴӦ����������������Ϊx������������Ӧ�Ľ�����������Ϊy��
Fe2O3��������![]()
Fe������Ϊ 1.12g
��������������ϡ���ᷴӦ����������������Ϊx
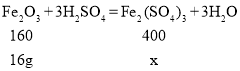
![]() x=40g
x=40g
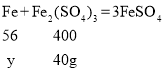
![]()
y=5.6g
5.6g��1.12g�����������٣����ܺ��ᷴӦ�ų�����
��������
����һ����1�����װ�õ������ԣ���C���ܽ���װ��ˮ���ձ��У��ȼ�װ�ã�װ���ڵ������������ͣ���ӵ��ܿ��ݳ�����˵�����������õ������ǣ�C���ܿ������ݣ�
��2����Ӧ��ʼ��������ֽ���������������ʷ�Ӧ�����ǣ������������ݣ�
�ڸ÷�Ӧ�У����������Ǵ�������MnO2�������Ǵ�����
��3��A����˿ֻ�������Ӵ�����Ȼ������B����˿��������ˮ�Ӵ�������Ұ�����ʵ������˵����������Ҫ��ˮ�������йأ�
B����˿��������ˮ�Ӵ�������Ұ���D����˿�������ˮ�Ӵ�����Ȼ������ʵ������Ա�˵�������������������һ����Ҫ������������Ũ�ȣ�����Ũ��Խ���������ٶ�Խ�죻
���������1�����������غ㶨�ɣ���Ӧ���ɵ�CO2��ˮ������������Ϊ102.24g-100.00g=2.24g��Dװ���м�ʯ�ҵ����������տ����еĶ�����̼��ˮ����ֹ�����еĶ�����̼��ˮ����װ��C�У�Ӱ��ʵ������
��2����������ʵ�����ݣ�ֻ�ܵõ����ɶ�����̼��ˮ������������������ɣ�����B��C֮������ϴ��ƿ������ʢ��Ũ���ᣬŨ���������ˮ�ԣ���������ˮ������ͨ��Ũ���������ı仯���ⶨ����ˮ��������Ȼ�����ˮ�������ó�Fe2O3H2O���������ٸ���Cװ�õ������仯���ó����ɶ�����̼���������̶��ó�FeCO3�����������ϴ��ƿ��Ũ���
��3�������Ľ����m��H2O��=1.80g��m��CO2��=0.44g��
������������Fe2O3H2O������Ϊx
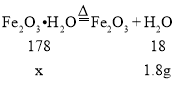
![]() x=17.8g
x=17.8g
������������FeCO3������Ϊy
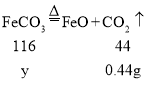
![]() y=1.16g
y=1.16g
���������е�����������Ϊ��20.08g-17.8g-1.16g=1.12g
������������Ϊ![]() ��
��
��4����20.08g������������������ϡ���ᣬ��ַ�Ӧ������������û��H2��
������̣�
�����������ᷴӦ����������������Ϊx������������Ӧ�Ľ�����������Ϊy��
Fe2O3��������![]() Fe������Ϊ 1.12g
Fe������Ϊ 1.12g
��������������ϡ���ᷴӦ����������������Ϊx
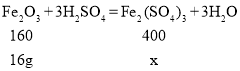
![]() x=40g
x=40g
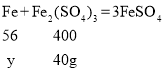
![]()
y=5.6g
5.6g��1.12g�����������٣����ܺ��ᷴӦ�ų�����������ޡ�