��Ŀ����
����Ŀ����۱�ʶ����̽�����о���ѧ����Ҫ�������ֶ�֮һ��
��1���������ʵ������з��ӡ�ԭ�Ӻ�___________��
��2�����۵ĽǶȿ���������������ԭ�ӹ��ɣ����ƶϳ���������________��ɣ�
��3��������֪����ͨ���Ժ������Ĺ۲졢˼����������������������ӵ���ʶ��
�ٽ���īˮ����ˮ�У�Ѹ����ɢ��˵��������_____________��
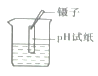
�ڽ�ͭ˿������������Һ�У��۲쵽��Һ��Ϊ��ɫ��ͭ˿����������İ�ɫ���ƶ���Һ������������˱仯����д����ѧ��Ӧ����ʽ_______________��
��4�����H2O��H2O2��Ԫ��������ͬ�����ӽṹ��ͬ�������ƶ϶��ߵĻ�ѧ���ʲ�ͬ����д��ʵ���ҷֽ�H2O2�������Ļ�ѧ����ʽ______________��
���𰸡����� ��Ԫ�� �����˶� Cu+2AgNO3=Cu(NO3)2+2Ag 2H2O2![]() 2H2O+O2��
2H2O+O2��
��������
��1���������ʵ����������Ƿ��ӡ�ԭ�ӡ����ӣ�������ӣ�
��2������������������ԭ�ӹ��ɵģ�������ֻ������Ԫ�أ�����˵����������Ԫ����ɵģ������Ԫ�أ�
��3���ٽ���īˮ����ˮ�У�Ѹ����ɢ��˵�������ڲ����˶�����������˶���
�ڽ�ͭ˿������������Һ�У�ͭ������������Ӧ��������ͭ���������Ի�ѧ����ʽΪ��Cu+2AgNO3=Cu(NO3)2+2Ag
��4��ʵ�����ù�������������ʱ��������ˮ�����������Ի�ѧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij�������÷������Ʊ�����ص��������£���ش���������:
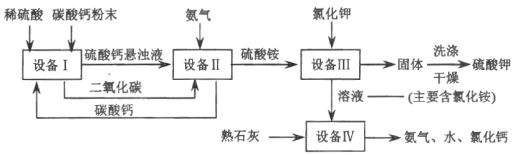
����:��֪20��ʱ����李�������ܽ�����±�:
���� | ����� | �Ȼ��� | �Ȼ�� | ����� |
�ܽ��/g(20��) | 11.1 | 34.2 | 37.2 | 75.4 |
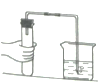
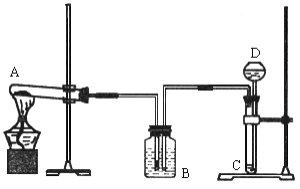
(1)���豸I���н�̼����гɷ�ĩ��Ŀ����___________��
(2)���豸II���з����Ļ�ѧ��Ӧ����ʽ��____________������______�����ͨ����������ͨ������̼����
(3)���豸III���з����Ļ�ѧ��Ӧ����ʽ��___________��
ϴ�Ӵӡ��豸III���еõ��Ĺ���ʱ������ˮ�����ñ����������Һ����Ŀ����________��
(4)���������п�ѭ��ʹ�õ����ʳ�ˮ�⣬����___________(��д��ѧʽ).