��Ŀ����
�������ƣ�CaO2���㷺Ӧ����ˮ����ֳ����ˮ�����������������Ĺ�������
�Ʊ�CaO2����ҵ�Ʊ��������Ƶ�һ�ַ������������Ʒ���
��1��Ca(OH)2��H2O2����ˮ���ڵ�����������CaO2·8H2O������120��ʱ��ȫ�ֽ�ΪCaO2�ȡ��йػ�ѧ��Ӧ����ʽΪ�� �� ��
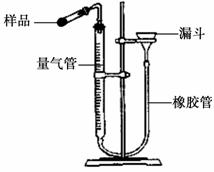 �ⶨCaO2���ȣ�CaO2��350��ʱ��Ѹ�ٷֽ⣬����CaO��O2����ͼ��ʵ���Ҳⶨ��Ʒ��CaO2������װ�á�
�ⶨCaO2���ȣ�CaO2��350��ʱ��Ѹ�ٷֽ⣬����CaO��O2����ͼ��ʵ���Ҳⶨ��Ʒ��CaO2������װ�á�
��2�����װ�������Եķ����ǣ����Ӻ�װ�ã���©��
עˮ�������������γɸ߶Ȳ���ñ�ǣ�һ��
ʱ��߶Ȳ� ��˵�����������á�
��3����ȷ��ȡ��������Һ��ij�ʼ�����ն���ǰ����
���еIJ����� ��
��4������С�Թ�ʱ�����Ź������Ʒֽ⣬�������ڵ�
Һ�����½���Ϊ��ֹ�Թܺ�������������ѹǿ
���ɽ�©�� ������ᡱ�����ơ�����
��5�����㺬����Ҫ�ⶨ�������� ��������ĸ��ţ�
A. ��������� B. ��Ʒ������ C. ��Ӧ��ʱ��
��6������Ʒ����Ϊ0.20 g����Ӧǰ�����ܶ���Ϊ2.10 mL����Ӧ�������ܶ���Ϊ24.50 mL���������������ܶ�Ϊ1.429g/L����ʵ���������ܵ���ѹ���� ��������ĸ��ţ�
A��50 mL B��100 mL C��1 L
��7����Ʒ��CaO2����Ϊ ��
��8������Ʒ������w��ʾ����Ӧǰ����ҩƷ��С�Թ�������Ϊm g����Ӧ���ڿ�������ȴ������ҩƷ��С�Թ�������Ϊn g����CaO2����= ���˷����ⶨ���ƫС��ԭ������� ��
���˷����ⶨ���ƫС��ԭ������� ��
���𰸡�
��1��Ca(OH)2+H2O2+6H2O= CaO2·8H2O
CaO2·8H2O CaO2+8H2O
CaO2+8H2O
��2������ ��3����������Һ����ƽ ��4������ ��5��AB ��6��A
��7��72%
��8���ڿ�������ȴ����CaO����ˮ��
��9�֣�ÿ��1�֣�



 ��2009?Ȫ�����ʼ죩��ĩ��С����ְ־���һ�����ߣ���������ʦ������������һ����ɫ�Ĺ��壬�����ж�ʱ�����������ݣ�����ѯ��֪�����ֹ����׳ơ��㸡�顱����Ҫ�ɷ��ǹ������ƣ�CaO2����
��2009?Ȫ�����ʼ죩��ĩ��С����ְ־���һ�����ߣ���������ʦ������������һ����ɫ�Ĺ��壬�����ж�ʱ�����������ݣ�����ѯ��֪�����ֹ����׳ơ��㸡�顱����Ҫ�ɷ��ǹ������ƣ�CaO2����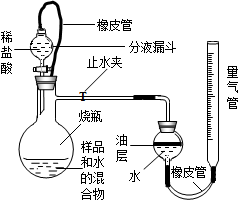 ��2013?�簲����ģ����;��������ʱ�����dz���ˮ�м��������������ƣ�CaO2�����壬Ϊ���ṩ����������������ˮ��Ӧ�����������⣬������ʲô���ʣ�������ȤС���������һ�����н���̽����������룮
��2013?�簲����ģ����;��������ʱ�����dz���ˮ�м��������������ƣ�CaO2�����壬Ϊ���ṩ����������������ˮ��Ӧ�����������⣬������ʲô���ʣ�������ȤС���������һ�����н���̽����������룮