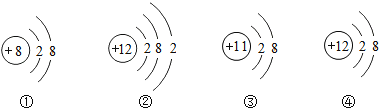
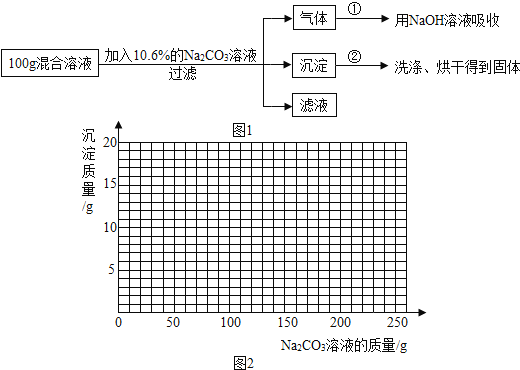
��Ŀ����
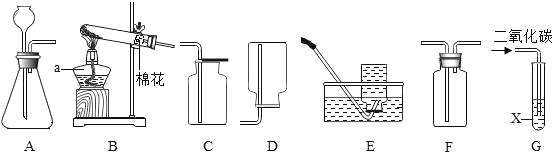
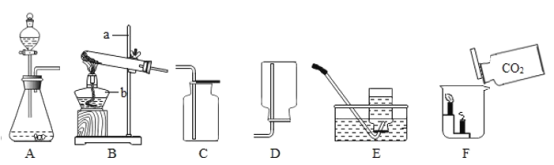
����Ŀ����ѧ��ȤС��Ϊ��֤�����غ㶨�ɣ�����þ���ڿ�����ȼ�յ�ʵ�飨ͼһ����
��1����д��þ����������Ӧ�Ļ�ѧ����ʽ_____________��
��2������������ȼ�ղ��������_______��������������С����������������þ����������������֪������һ�����ԭ����_________��
��3����ȤС�鰴ͼ��װ�øĽ�ʵ�����֤�������غ㶨�ɣ�ͬʱ����ȼ�ղ�������������ɫ���塣
��������⣩��ɫ������ʲô�أ�
���������ϣ�������þΪ��ɫ���壬��������ˮ����Ӧ����þ���뵪����Ӧ���ɻ�ɫ�ĵ���þ��Mg3N2�����壻�۵���þ����ˮ���ҷ�Ӧ������������������ʹʪ��ĺ�ɫʯ����ֽ������
���������룩��ɫ����ΪMg3N2
������ʵ�飩ͬѧ��ͨ��ʵ��Բ����������֤�����㽫ʵ���¼����������
ʵ����� | ʵ������ | ���� |
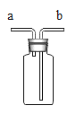
��þ��ȼ�յIJ������һֻ�Թ��У���������������ˮ��Ȼ��һʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲����� | _______ | ��ɫ���� Ϊ����þ |
����˼�뽻���������е����ĺ���Զ���������ĺ�������þ���ڿ�����ȼ�����ɵ�����þȴԶ���ڵ���þ������Ϊ ___________��þ��_________��������������������������������ⶨ����������������ʵ�飬��Ϊ________________��
���𰸡�2Mg+O2![]() 2MgO ���� ��Ӧ�����ж��˲μӷ�Ӧ����������������˼�Լ��ɣ� ʪ��ĺ�ɫʯ����ֽ���� �����Ļ�ѧ���ʱȵ������� ���� þ��������������е�������Ӧ�������뵪����Ӧ
2MgO ���� ��Ӧ�����ж��˲μӷ�Ӧ����������������˼�Լ��ɣ� ʪ��ĺ�ɫʯ����ֽ���� �����Ļ�ѧ���ʱȵ������� ���� þ��������������е�������Ӧ�������뵪����Ӧ
��������
��1��þ����������Ӧ��������þ����Ӧ�Ļ�ѧ����ʽΪ��2Mg+O2![]() 2MgO��
2MgO��
��2������������ȼ�ղ�����������ڷ�Ӧ��þ������������Ϊ�����е�������þ��Ӧ����������þ����Ӧ�����ж��˲μӷ�Ӧ��������������
��3��[����ʵ��]��һ�����ĵ���þ�����Թ��У�Ȼ�����������ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ͽ����ǣ��д�������ð����ʪ��ĺ�ɫʯ����ֽ��������˻�ɫ������Mg3N2��
[��˼�뽻��]
������N2�ĺ���Զ����O2�ĺ�������þ���ڿ�����ȼ�����ɵ�MgO������ȴ����Mg3N2�������Ľ����ǣ������ȵ������ã���ͬ�������Ժ�������ӦΪ����
þ�����ܴ���������ⶨ����������������ʵ�飬��Ϊþ��������������е�������Ӧ�������뵪����Ӧ��

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�����Ŀ���±���NaCl��KNO3�����ڲ�ͬ�¶��µIJ����ܽ�����ݣ���λ��g/100gˮ������ش����⣺
�¶ȣ����� | 10 | 20 | 30 | 40 | 60 |
NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.3 |
KNO3 | 20.9 | 31.6 | 45.8 | 63.9 | 110 |
��1��20��ʱ��NaCl���ܽ����_____g/100gˮ��
��2���������������ܽ�ȱ仯���¶�Ӱ���С����_____��ѡ����NaCl������KNO3������
��3��20��ʱ����20gNaCl�������50gˮ�У���ֽ��裬�γɵ���Һ����Ϊ_____g��
��4��Ϊ�˽���������NaCl���ʵ�KNO3�ı�����Һ�ᴿ����ͨ��_____������ýϴ�����KNO3���壻
��5��40��ʱ����NaCl��KNO3�ı�����Һ��100g���µ�20������ʣ����Һ������ȷ����_____��
A ���ʵ�����������NaCl��KNO3
B ���������������NaCl��KNO3
C NaCl��KNO3���DZ�����Һ
D �ܼ���������NaCl��KNO3