��Ŀ����
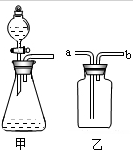
ijУ�����뻯ѧ���С��ͨ�����������˽���ҹ�����ÿ������Լ40������ϴ����ٶ���Щʹ�ú�ķ����ϴ���ͨ�����մ��������ŷŴ���CO2���ûС������ͼ��ʾװ�ã�����һ�����������ϴ�ȼ�պ����CO2��������

(1)Ϊ�˱�֤������ȷ�ԣ����Ӻ�ʵ��װ�ú�Ӧ���________________���ټ���ҩƷ����ʵ�顣
(2)װ�â��С�ձ��м�������H2O2�ʹ������ɳ�������O2�����ϴ����ȼ�գ�����O2�Ļ�ѧ����ʽ�ǣ�________________________________________________��
(3)���ϴ�ȼ����װ�â�����ȴʱ��װ�â���Һ��Ҳ����������װ�â��У�ԭ����__________________________________��
(4)����ʵ��Ŀ�ģ���Ҫ��������______(����ĸ)��
A��ʵ��ǰ���ϴ�������
B��ʵ��ǰ��ʵ���װ�â������
C��ʵ��ǰ��ʵ���װ�â������

(1)Ϊ�˱�֤������ȷ�ԣ����Ӻ�ʵ��װ�ú�Ӧ���________________���ټ���ҩƷ����ʵ�顣
(2)װ�â��С�ձ��м�������H2O2�ʹ������ɳ�������O2�����ϴ����ȼ�գ�����O2�Ļ�ѧ����ʽ�ǣ�________________________________________________��
(3)���ϴ�ȼ����װ�â�����ȴʱ��װ�â���Һ��Ҳ����������װ�â��У�ԭ����__________________________________��
(4)����ʵ��Ŀ�ģ���Ҫ��������______(����ĸ)��
A��ʵ��ǰ���ϴ�������
B��ʵ��ǰ��ʵ���װ�â������
C��ʵ��ǰ��ʵ���װ�â������
(1)װ�������ԡ� (2)2H2O2 2H2O��O2��
2H2O��O2��
(3)װ�â��в�������������ʹѹǿ���� (4)AC
 2H2O��O2��
2H2O��O2��(3)װ�â��в�������������ʹѹǿ���� (4)AC
�����������ʵ�鿪ʼʱһ��Ҫ����װ�õ������ԣ����������ڶ������̵Ĵ�������������ˮ��������Ϊ�˲���һ���������ϴ�ȼ�պ������CO2����������Ҫ֪�����Ϻ����ɵĶ�����̼��������
��1��Ϊ�˱�֤������ȷ�ԣ����Ӻ�ʵ��װ�ú�Ӧ���װ�������ԣ��ټ���ҩƷ����ʵ�飮���װ�������ԣ�
��2����������ֽ����������Ļ�ѧ����ʽΪ��2H2O2
 2H2O��O2��
2H2O��O2����3��װ�â�����ȴʱ��װ�â���Һ��Ҳ����������װ�â��У�ԭ����װ�â�������������ʹ����ѹǿ�����װ�â�������������ʹ����ѹǿ����
��4������ʵ��Ŀ�ģ�ʵ��Ŀ���ǡ�һ���������ϴ�ȼ�պ������CO2����������Ҳ����Ҫ֪�����ϺͶ�����̼������������ϵ��������Ҫ��������ʵ��ǰ���ϴ���������ʵ��ǰ��ʵ���װ�â�����������AC��
������Ҫ���������غ㶨������д��ѧ����ʽ��Ҫ����ʵ��Ŀ��ѡ����ʵ����ݽ��н��С��жϣ��Ӷ��ó���ȷ�Ľ��ۣ�

��ϰ��ϵ�д�
�����Ŀ