��Ŀ����
����Ŀ����Դ�������������������ᷢչ������ء�
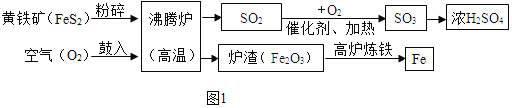
��1��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ�________.
��2��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ�����壬�˹��̿���Ϊ��̼��ˮ�����ڸ��������µķ�Ӧ������ʾ��ͼ������ʾ��
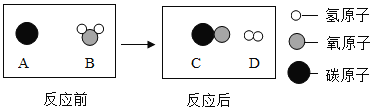
�÷�Ӧ�Ļ�ѧ����ʽΪ_________��������Ӧ����Ϊ________��
��3��Ϊ�������������ŷţ����ǻ���Ѱ�Ҳ���̼Ԫ�ص�ȼ�ϡ����о�����![]() ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ����
ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ����![]() �е�Ԫ�غ���Ԫ�ص�������Ϊ________��
�е�Ԫ�غ���Ԫ�ص�������Ϊ________��![]() ȼ�����ɿ����к������������ˮ����д���˷�Ӧ�Ļ�ѧ����ʽ__________��
ȼ�����ɿ����к������������ˮ����д���˷�Ӧ�Ļ�ѧ����ʽ__________��
���𰸡���Ȼ�� C+H2O![]() CO+H2 �û���Ӧ 14��3 4NH3+3O2
CO+H2 �û���Ӧ 14��3 4NH3+3O2![]() 2N2+6H2O
2N2+6H2O
��������
��1�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ���������Ȼ����
��2��������ģ��ͼ��֪��̼��ˮ�ڸ����·�Ӧ����һ����̼���������˷�Ӧ�ǵ��ʺͻ����ﷴӦ���ɵ��ʺͻ�����������û���Ӧ�����C+H2O![]() CO+H2 �û���Ӧ��
CO+H2 �û���Ӧ��
��3��![]() �е�Ԫ�غ���Ԫ�ص�������Ϊ14����1��3��=14��3��
�е�Ԫ�غ���Ԫ�ص�������Ϊ14����1��3��=14��3��![]() ȼ�����ɿ����к������������ˮ�������к������������ǵ��������Դ˷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+3O2
ȼ�����ɿ����к������������ˮ�������к������������ǵ��������Դ˷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+3O2![]() 2N2+6H2O�����14��3 4NH3+3O2
2N2+6H2O�����14��3 4NH3+3O2![]() 2N2+6H2O��
2N2+6H2O��