��Ŀ����
����Ŀ���������Ļ������ڹ�ũҵ�������й㷺��Ӧ�á�
(һ)����Ӧ�úͷ���
A���ҹ�������ʱ�ھ��������������Ϊͭ��֮˵���û�ѧ����ʽ��ʾ��ԭ����_____��
B�����������Ҫ����Ϊ��
Fe![]() Fe(OH)2
Fe(OH)2![]() Fe(OH)3
Fe(OH)3![]() Fe2O3xH2O
Fe2O3xH2O
(1)д��ת���ٵĻ�ѧ����ʽ_____��
(2)ת�������� Fe2O3xH2O���� x��_____(x Ϊ����)��
C.�������ֹ�������һ����ʩ_____��
(��)���Ļ�����Ӧ��
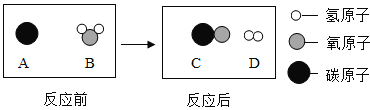
������(��Ҫ�ɷ���FeS2)����һ����Ҫ�Ļ���ԭ�ϣ��������Ʊ����������(ͼ1)��
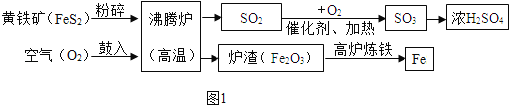
A����ҵ�Ͻ�����������Ŀ����_____��
B������������β���к��� SO2����ֱ���ŷſ��ܻ���ɻ���������_____��
C����¯�����Ļ�ѧ����ʽΪ_____��
D.150 �ֺ� FeS280%�Ļ����������������Ƶ� 98%��Ũ����_____�֡�
(��)����ұ����̽��
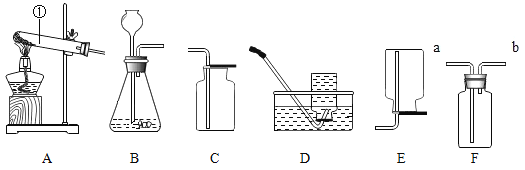
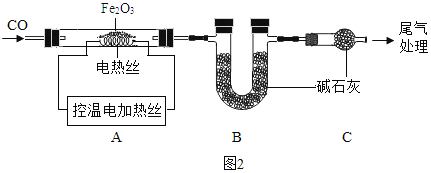
ȡ 24.0g Fe2O3 ��ĩ��С��ͬѧ��ͼ2װ��ģ������������÷�Ӧ�����ɷ֡�
���ϣ���ʯ�ҿ����� H2O �� CO2��
A������װ�ã���_____����װ��ҩƷ��
B��ʵ��ʱ��ͨ��CO��Ŀ����_____��
C������A���¶��� 700��������ȫ����ڣ�����ͨCO����ȴ��
(1)ͨ���ⶨװ�� B �й���������仯����ȷ���װ�� A ��ʣ�����������װ��C��������_____��
(2)��ֱ�Ӳ��װ�� A ��ʣ���������Ϊ19.2g����װ�� B �й���Ӧ����_____g��
D��������A��ʣ����� 19.2g ΪFe�� FexOy �Ļ��������м���������ϡ H2SO4��� ��Ӧ���� H20.3g��
(1)������ Fe ������Ϊ_____g��
(2)FexOy �Ļ�ѧʽΪ_____��
���𰸡� Fe+CuSO4=Cu+FeSO4 2Fe+O2+2H2O�T2Fe(OH)2 2 ������Ʒ����Ϳ������ ����Ӧ��ĽӴ���� ���� Fe2O3+3CO![]() 2Fe+3CO2 200 ���װ�õ������� �ž�װ���еĿ��� ���տ����еĶ�����̼��ˮ���� 13.2 8.4 Fe2O3
2Fe+3CO2 200 ���װ�õ������� �ž�װ���еĿ��� ���տ����еĶ�����̼��ˮ���� 13.2 8.4 Fe2O3
�����������������Ϊͭ��ָ��������ͭ��Ӧ��������������ͭ���ʷ�Ӧ����ʽΪFe+CuSO4=Cu+FeSO4��
(1)ת����������������ˮ��Ӧ����������������Ӧ�ķ���ʽΪ2Fe+O2+2H2O�T2Fe(OH)2��
(2)������������Fe2O3xH2O �ķ�Ӧ����ʽΪ2Fe(OH)3![]() Fe2O3xH2O+��3-x��H2O����x��2�� ��ֹ��������ķ�����������Ʒ����Ϳ������ȣ�
Fe2O3xH2O+��3-x��H2O����x��2�� ��ֹ��������ķ�����������Ʒ����Ϳ������ȣ�
������A����ҵ�Ͻ�����������Ŀ��������Ӧ��ĽӴ������
B������������β���к��� SO2����ֱ���ŷſ��ܻ���ɻ������������ꣻ
C����¯������̼��������Ӧ���ɶ�����̼��������̼��̼��Ӧ����һ����̼��һ����̼������ͭ��Ӧ�������Ͷ�����̼����Ӧ����ʽΪFe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��
D.��Ԫ���غ�ɵù�ϵʽFeS2-- 2SO2��2H2SO4��150 �ֺ� FeS280%�Ļ����������98%��Ũ���������Ϊx
FeS2------- 2SO2-------2H2SO4
120 196
150t![]() x
x![]()
![]() =
=![]() �����x=200t
�����x=200t
(��)
A����ʵ�������������ɣ���ʵ��ǰҪ���װ�õ������ԣ�
B��һ����̼�ǿ�ȼ�����壬��һ����̼�к���������ȼʱ������ ��ը����ʵ��ʱ��ͨ��CO���ž����еĿ�������ֹ��ը��
C��(1)���������װ��B��Ӱ��ʵ��Ľ������װ��C�����տ����еĶ�����̼��ˮ������
��2����÷�Ӧ�����ɶ�����̼������Ϊx
Fe2O3+3CO![]() 2Fe+3CO2 ������ٵ���
2Fe+3CO2 ������ٵ���
132 48
x 24g-19.2g
![]() =
=![]() �����x=13.2g
�����x=13.2g
D����1��������0.3g H2��ҪFe������Ϊx��
Fe+H2SO4= H2![]() +FeSO4
+FeSO4
56 2
x 0.3g
![]() =
=![]() �����x=8.4g
�����x=8.4g
��2��19.2g��ʣ���������������Ϊ8.4g�������Ĺ�����û�з�Ӧ������������ѧʽΪFe2O3��

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�