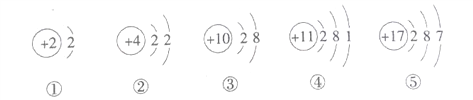
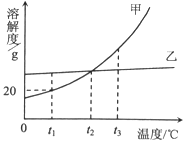
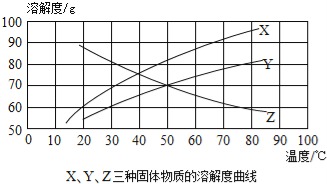
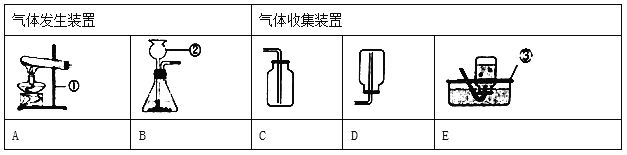
��Ŀ����
����Ŀ������ͭ����ͭ��п�Ͻ�Ϊ�˲ⶨij��ͭ��Ʒ��ͭ�������������ס��ҡ�����λͬѧ�ֱ����ʵ�飬ʵ���������£����ձ�����Ϊ56 g��
�� | �� | �� | |
���ձ�+ϡ���ᣩ���� | 139 g | 129 g | 129 g |
�����ͭ��Ʒ���� | 20 g | 24 g | 20 g |
��ַ�Ӧ���ձ�+ʣ�����ʣ����� | 158.8 g | 152.8 g | 148.8 g |
��1��ʵ��֤����λͬѧ�У�ֻ��һλͬѧȡ�õ�ϡ�������Ʒǡ����ȫ��Ӧ�����ͬѧ����λͬѧ��
��2������㣺��ͭ��Ʒ��ͭ������������Zn + 2HCl ===== Zn Cl2 + H2��
���𰸡���������67.5��
���������⣺(1)�ס��ҡ������ηų���������������0.2g������ϡ����������࣬���л�ͭ��Ʒ�����࣬���Ա�ͬѧȡ�õ�ϡ�������Ʒǡ����ȫ��Ӧ������ȡ��������������129g+20g-148.8g=0.2g��(2)�裺��ͭ��Ʒ�вμӷ�Ӧп������Ϊx��
Zn+2HCl�TZnCl2+H2��
65 2
x 0.2g
![]()
x=6.5g
��ͭ��Ʒ��ͭ����������=![]() ��100%=67.5%��
��100%=67.5%��
��(1)��ͬѧȡ�õ�ϡ�������Ʒǡ����ȫ��Ӧ��(2)��ͭ��Ʒ��ͭ����������Ϊ67.5%��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ