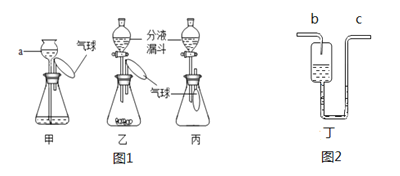
��Ŀ����
����Ŀ����ѧ���츣����Ŀ�ѧ��


(1)��Դ��ѧ����2017��5��18�գ��й���ѧ���״����Ϻ��Բɿ�ȼ��ȡ��Բ���ɹ���ʵ�����ҹ���Ȼ��ˮ���↑������ʷ��ͻ�ơ���ȼ����һ�����������Դ���仯ѧʽΪCH48H2O����ȼ����ȫȼ�յĻ�ѧ����ʽ��______________��������
(2)ũҵ��ѧ������Ԥ����궬�������ɺ�������ֲ�����Ĺ�ũ��Ӧ�Թ��߲����ʣ����������У������ʵ���__________(����ĸ���)��
A��CO(NH2)2 B��K2SO4 �� ��C��Ca(H2PO4)2 D�� KNO3
(3)���ϻ�ѧ�������������г�������Ʒ�У����л��ϳɲ������ɵ���______(����ĸ���)��
A�����Ͻ������� B����˯�� C���մɻ�ƿ D����������
(4)��ҵ��ѧ�������������������������зdz���Ҫ�IJ��ϣ���ÿ������ʴ����ʧ�������Ǿ�ģ���д��һ����ֹ������ʴ��ʩ_____________________��
(5)�Ƽ���ѧ����2017��4��20��19ʱ41�֡�����һ�š����ػ���������ա�������һ�š����ػ����ȼ��Ϊ���塣����ȼ�������ۺ������(��ѧʽΪ��NH4C1O4)�Ļ�������ʱ����ȼ���۲���������������������立������·�Ӧ�� 2NH4C1O4 =N2��+2O2��+ Cl2��+4X��������X�Ļ�ѧʽΪ________��
���𰸡� 2CH48H2O+2O2![]() CO2 +10H2O�� C D ��ơ�Ϳ����Ⱦ��� H2O
CO2 +10H2O�� C D ��ơ�Ϳ����Ⱦ��� H2O
��������(1)�״���ȫȼ�����ɶ�����̼��ˮ����ѧ����ʽΪ��2CH48H2O+2O2![]() CO2 +10H2O��(2)A��CO��NH2��2�к��е�Ԫ�أ����ڵ��ʣ�B��K2SO4���м�Ԫ�أ����ڼطʣ�C��Ca��H2PO4��2������Ԫ�أ������ʣ�D��K2NO3���м�Ԫ�أ����ڼط�����ѡC��(3) A�����Ͻ����ڽ������ϣ�B������������Ȼ���ϣ�C���մɻ�ƿ�������ǽ������ϣ�D�����������л��ϳɲ��ϣ����D��(4) ��ֹ������ʴ��ʩ�У���ơ�Ϳ����ȣ�(5)���������غ㶨�ɣ���ϻ�ѧ����ʽ����Ӧǰ��Ԫ�ص�����䣬ԭ�Ӹ������䣺��Ӧ�2����ԭ�ӡ�2����ԭ�ӡ�8����ԭ�ӡ�8����ԭ�ӣ������N2��2O2��Cl2����2����ԭ�ӡ�4����ԭ�ӡ�2����ԭ�ӡ��ʡ�4X������8����ԭ�ӡ�4����ԭ�ӣ��ʻ�ѧʽΪH2O��
CO2 +10H2O��(2)A��CO��NH2��2�к��е�Ԫ�أ����ڵ��ʣ�B��K2SO4���м�Ԫ�أ����ڼطʣ�C��Ca��H2PO4��2������Ԫ�أ������ʣ�D��K2NO3���м�Ԫ�أ����ڼط�����ѡC��(3) A�����Ͻ����ڽ������ϣ�B������������Ȼ���ϣ�C���մɻ�ƿ�������ǽ������ϣ�D�����������л��ϳɲ��ϣ����D��(4) ��ֹ������ʴ��ʩ�У���ơ�Ϳ����ȣ�(5)���������غ㶨�ɣ���ϻ�ѧ����ʽ����Ӧǰ��Ԫ�ص�����䣬ԭ�Ӹ������䣺��Ӧ�2����ԭ�ӡ�2����ԭ�ӡ�8����ԭ�ӡ�8����ԭ�ӣ������N2��2O2��Cl2����2����ԭ�ӡ�4����ԭ�ӡ�2����ԭ�ӡ��ʡ�4X������8����ԭ�ӡ�4����ԭ�ӣ��ʻ�ѧʽΪH2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ҵ������Ҫ�ɷ���Fe2O3��������������FeO��Fe3O4
���������ϡ�(1)���ᾧ��(H2C2O43H2O)��Ũ�������������ȷֽ⣬��ѧ����ʽΪ��H2C2O43H2O ![]() CO2��+CO��+H2O
CO2��+CO��+H2O
(2)��ʯ���ǹ���NaOH��CaO�Ļ���������ˮ�����Ͷ�����̼��
(3)���ij�������������������������
���������� | FeO | Fe2O3 | Fe3O4 |
������������ | 77.8% | 70.0% | 72.4% |
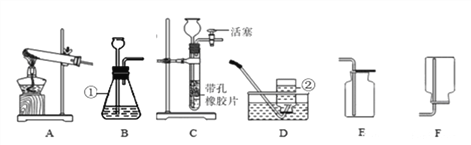
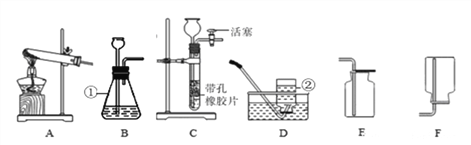
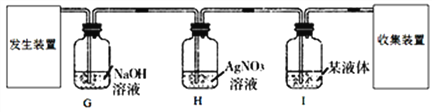
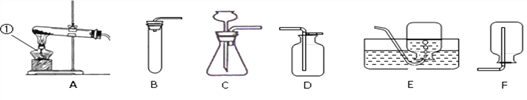
���������ۡ�Ϊ�˲ⶨ��������������������С����������ʵ�顣(װ������������)
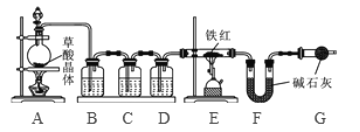
(1)��ʵ��Ϊ�˱�֤����E�е������Ǵ����������CO����B��C��D�е��Լ�������________(����ĸ���)
a��Ũ���� b�������ʯ��ˮ c����������Һ
(2)Cװ�õ������� __________________________��
(3)д��Eװ������������Ӧ��һ����ѧ����ʽ�� _____________________��
(4)��ȡ������Ʒ10.0g,������װ�ý���ʵ�飬�ⶨ��������������������
����E�г�ַ�Ӧ��õ����۵�����Ϊmg���� ____ < m < ______��
����ʵ��ǰ��Ƶ�Fװ�É���7.7g������������������������� _________��
��ʵ�鷴˼�� (1)���ȱ��Gװ��(��������������)��������Ʒ���������������� ________(ѡ����ƫС������������ƫ����)��
(2)��ʵ��װ�õ�һ������ȱ���� ___________________��