��Ŀ����
����Ŀ��������ͼ��ʾװ�ûش����⣺
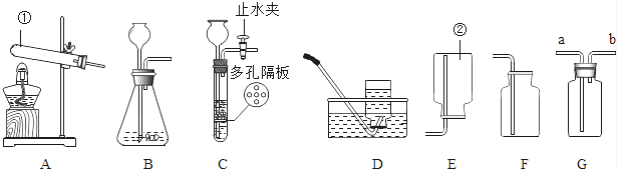
��1��д���������������ƣ���________����________��
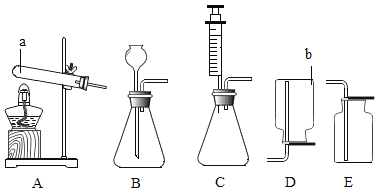
��2�����������ȡ������ѡ��ķ���װ���ǡ�________����Ӧ�Ļ�ѧ����ʽΪ________��
��3��ʵ��������Bװ������ȡ������̼ʱ��������©����Ӧ����________������G���ռ�������̼��������̼Ӧ�ӡ�________����ѡ��a����b��������ȡ������̼���ѡ��ķ���װ����________��ѡ��B����C����������һ��װ����ȣ����ŵ��ǣ�________ ��
��4��������һ����ɫ���г�������ζ�����壬������ˮ��ʵ��������������ϡ���ᷴӦ��ȡ�������壮�Իش�
������������г�������ζ�����������________�����������ѧ�������ʣ�
��ʵ������ȡH2S����ʱ������װ��Ӧѡ��________���ռ�װ��Ӧѡ��________����������ţ���
���𰸡��Թܼ���ƿA2KClO3![]() 2KCl+3O2��ϡ����aC���Կ��Ʒ�Ӧ�Ŀ�ʼ��������濪���ã������ͣ������B��CF
2KCl+3O2��ϡ����aC���Կ��Ʒ�Ӧ�Ŀ�ʼ��������濪���ã������ͣ������B��CF
��������
��1�������ٵ��������Թܣ������ڵ������Ǽ���ƿ��
��2�����������ȡ������Ҫ���м��ȣ���ѡ��ķ���װ����A����Ӧ�Ļ�ѧ����ʽΪ2KClO3![]() 2KCl+3O2����
2KCl+3O2����
��3��ʵ������Bװ�ã����ô���ʯ��ʯ��ʯ����Ҫ�ɷ���̼��ƣ���ϡ���ᷴӦ����ȡ������̼����������©����Ӧ����ϡ���������̼�ܶȴ��ڿ���������G���ռ�������̼��������̼Ӧ��a������ȡ������̼���ѡ��ķ���װ����C���������Կ��Ʒ�Ӧ�Ŀ�ʼ�ͽ�����
��4��
������������г�������ζ�������ʲ���Ҫͨ����ѧ�仯���б��֣��������������ʣ�
��ʵ������ȡH2S���壬Ϊ��Һ���·�Ӧ���ʷ���װ�ÿ�ѡ��B��C���������Է�������Ϊ34��������ƽ����Է�������Ϊ29����������ܶȴ��ڿ�������������ˮ����ѡ�������ſ��������ռ�װ��ѡ��F��

 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�����Ŀ���ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ�����ᷢչ��Ƽ����������˾��ס��ϳɰ����յ���Ҫ�������£�

(1)�ϳ����еķ�Ӧ�����ڸ��¡���ѹ�����������½��У��÷�Ӧ�ķ��ű���ʽ�ǣ�__________________________________________________��
(2)���������п��ظ�ʹ�õ�������_______________(�ѧʽ)��
(3)���ݱ��е����ݻش����⡣
���� | H2 | N2 | O2 | NH3 |
�е�/��(1.01��105 Pa) | �C252 | �C195.8 | �C183 | �C33.35 |
��1.01��105 Paʱ��Ҫ������NH3��N2��H2���뿪���������˵��¶�Ӧ�ÿ�����______����
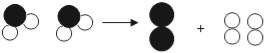
(4)��ͼ�Ǻϳ����з�����Ӧ��������ʾ��ͼ��
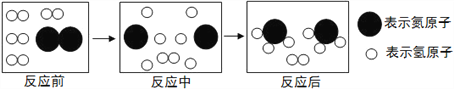
��ͼ��֪���ַ�Ӧ��N2��H2�ķ��Ӹ�����Ϊ____���÷�Ӧ�е���С������_________(��д��ѧ����)��
����Ŀ��ʵ������һƿ���ܲ������Լ�����ͼ1�������ȱ�ı�ǩ��ֻʣ����Na������10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
��������⣩��ƿ�Լ�������ʲô��Һ�أ�
���������ϣ�
�������л�ѧ�����ĺ��ƻ�������NaCl��NaOH��Na2CO3��NaHCO3 ��
��Na2CO3��NaHCO3��Һ���ʼ��ԣ�
�������£�20����ʱ���������ʵ��ܽ�ȵ��������£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�ܽ��g | 36 | 109 | 215 | 9.6 |
���ó����ۣ�С�������Լ�ƿ��ע��������������10%���ϱ��е��ܽ�ȵ������жϣ���ƿ�Լ���������________ ��
���������룩��������NaOH��Һ����������Na2CO3��Һ����������NaCl��
����Ʋ�ʵ�飩
��1��Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����PH��7�������________ ������
��2��СǿΪ��ȷ������Һ�ijɷݣ����ֽ���������ʵ�飺
�������� | ʵ������ | ���ۼ���ѧ����ʽ |
ȡ�����Թ��У��μ������� ________ �����Լ������ƣ� | �������������� | ��������ȷ |
��ʦָ���ý��۲����ܣ�����������������Һ�ڿ����г��ڷ��ûᷢ�����ʣ����ʺ�Ҳ�ܲ�������������д�����������ڿ����б��ʵĻ�ѧ����ʽ��________ ����
������̽������ȡ�����������CaCl2��Һ���۲쵽��________ �����������һ�����Ŀ����________ �����ú�ȡ�ϲ���Һ��������ɫ��̪��Һ����Һ�ʺ�ɫ��
��ʵ����ۣ���ƿ��Һԭ����________ ��
��̽����ʾ����ʵ��ʱȡ��ҩƷ��Ӧ________ ��
��3��̽����ƿNaOH��Һ�ı��ʳ̶�
���о�������ȡ10gԭ��Һ����������μ�����������Ϊ7.3%��ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȣ�
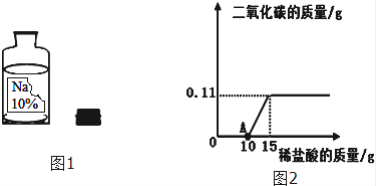
��������⣩ʵ���ü���ϡ���������CO2�����������ϵ��ͼ2��ʾ��
��ش���A����Һ�����������ʡ�________ ���ѧʽ����
��10gԭ��Һ�к�̼���Ƶ�����________ ����д��������̣�����2�֣�
��10gԭ��Һ��δ���ʵ��������Ƶ�����________ ��������Ҫд��������̣�