��Ŀ����
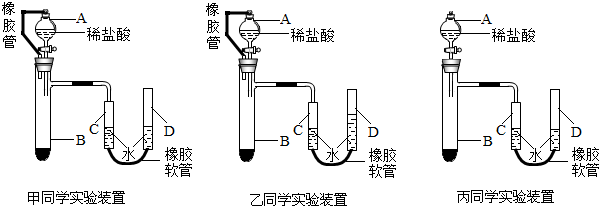
�ס��ҡ�����λͬѧ�������Լ��ֱ����ʵ�飬��ȡ�Լ��������±���ʾ���ҷ�Ӧ��ǡ����ȫ��
��֪��A1+A2+A3=23.0g��B1+B2+B3=219.0g���ֽ��ס��ҡ�����λͬѧ���õ�����Һ����ͬһ�����У�������Һ������Ϊ237.6g����
��1����ͬѧ��ȡCaCO3�����������
��2���������û����Һ�����ʵ�����������
| �Լ������� | ��Ӧ��������Һ������ | ||
| �� | CaO����A1g | 10%����B1g | C1g |
| �� | Ca��OH��2����A2g | 10%����B2g | C2g |
| �� | CaCO3����A3g | 10%����B3g | C3g |
��1����ͬѧ��ȡCaCO3�����������
��2���������û����Һ�����ʵ�����������
��1�����ͬѧ��ȡCaCO3���������Ϊx
��Ӧ���ɶ�����̼������Ϊ��23.0g+219.0g-237.6g=4.4g
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g
=
x=10.0g
��2���跴Ӧ������CaCl2������Ϊy
2HCl��2Cl��CaCl2
73 111
219g��10% y
=
y=33.3g
CaCl2����������=
��100%=14.0%
�𣺣�1����ͬѧ��ȡCaCO3���������Ϊ10.0g��
��2���������û����Һ�����ʵ���������Ϊ14.0%��
��Ӧ���ɶ�����̼������Ϊ��23.0g+219.0g-237.6g=4.4g
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g
| 100 |
| x |
| 44 |
| 4.4g |
x=10.0g
��2���跴Ӧ������CaCl2������Ϊy
2HCl��2Cl��CaCl2
73 111
219g��10% y
| 73 |
| 219g��10% |
| 111 |
| y |
CaCl2����������=
| 33.3g |
| 237.6g |
�𣺣�1����ͬѧ��ȡCaCO3���������Ϊ10.0g��
��2���������û����Һ�����ʵ���������Ϊ14.0%��

��ϰ��ϵ�д�
�����Ŀ
�ס��ҡ�����λͬѧ�������Լ��ֱ����ʵ�飬��ǡ����ȫ��Ӧ�������Լ����������±�
��֪a1+a2+a3=23.04g��b1+b2+b3=189.8g���ֽ��ס��ҡ�����ͬѧ������Һȫ������һ�������ڣ��Ƶ���ҺΪ206.16g������˻����Һ��������������������ȷ��0.1%��
| �Լ������� | ��Ӧ��������Һ���� | ||
| �� | CaO���̣�a1g | 10%����b1g | C1 |
| �� | Ca��OH��2���̣�a2g | 10%����b2g | C2 |
| �� | CaCO3���̣�a3g | 10%����b3g | C3 |