��Ŀ����
����Ŀ�������������ж��֣���Ȫˮƿ�����õ�������Ҫ�ɷ�Ϊֻ��̼����Ԫ�ص��л�����ȶ��������л�������������ʱȼ�ղ���Ϊ������̼��ˮ������������ʱȼ�ղ���Ϊ������̼��һ����̼��ˮ���ȶ��������뷴ӦҲ��������ij��ѧ�о�С���ͬѧ���Կ�Ȫˮƿ�ӵ���ɽ���̽�����������������ͼ��ʾ��ʵ��װ�ã���װ�����������á�
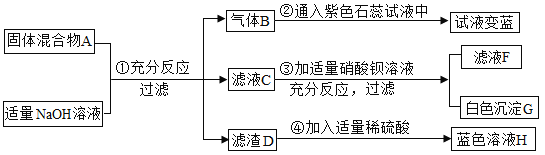
ʵ����Ҫ��������:
��.���װ�������Ժ�װ����������ƿ���Ͽ�������װ��
��.���з�Һ©��������ע��˫��ˮ�رջ���;
��.һ��ʱ������ӱ�����װ�ã���ȼ�ƾ��ƣ��۲쵽����ȼ�գ����ȼ�պ��װ���й��������
IV.Ϩ��ƾ��ƣ�����ͨ������ֱ����������ȴ��
ʵ��ⶨ��������:
���й��� | ��װ�� | ��װ�� | ��װ�� | |
ʵ��ǰ | 5.0g | 118.2g | 224.6g | 148.0g |
ʵ��� | m | 123.6g | 237.8g | 148.0g |
��1��������װҩƷ��_______����װ�õ�������________��
��2������IV��ͨ��������������__________��
��3������ʵ�鲻�ô�����������ͨ����������ݱ�����װ�������仯�����ʹ�ⶨ��C��HԪ�ص�������______(��ƫ��������ƫС��������Ӱ����)��ԭ����______��
��4��m��ֵΪ_______��
���𰸡�Ũ���� ���տ����еĶ�����̼��ˮ���� ʹ������м��ȫ��Ӧ,��������������������װ�ö����������� ƫ�� �����к���һ�����Ķ�����̼ 0.8g
��������
��1��Ũ���������ˮ�ԣ���ʯ�һ���ˮ��������̼��Ӧ�����Զ�����װҩƷ��Ũ���ᣬ��װ�õ������ǣ����տ����еĶ�����̼��ˮ��������ֹ��װ��β�˽���Ӱ��ʵ������
��2�����������ϻᷴӦ���ɶ�����̼��ˮ�����Բ�������ͨ�������������ǣ�ʹ������м��ȫ��Ӧ,��������������������װ�ö����������գ�
��3�������к���һ�����Ķ�����̼�����Ը�ʵ�鲻�ô�����������ͨ����������ݱ�����װ�������仯�����ʹ�ⶨ��C��HԪ�ص�������ƫ��ԭ���ǣ������к���һ�����Ķ�����̼��
��4��Ũ���������ˮ�ԣ�����ʯ��ˮ�������ն�����̼��
��װ�������ӵ���ˮ��������5.4g����װ�������ӵ�Ϊ������̼��������13.2g����������̼����Ԫ�ص�����Ϊ��5.4g��![]() +13.2g��
+13.2g��![]() =4.2g������m��ֵΪ5g-4.2g=0.8g��
=4.2g������m��ֵΪ5g-4.2g=0.8g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ȼ�������¼������������ʷ�����Ӧ�����ɱ����ʺͶ����ʣ����ǵ�����ͼ�ͷ�Ӧǰ����������±���ʾ��
������� | �� | �� | �� | �� |
|
��ʾ��ͼ |
|
|
|
| |
��Ӧǰ����/g | 68 | 100 | 1 | 0 | |
��Ӧ������/g | 0 | x | y | z |
��1����![]() ���ɵ������У�
���ɵ������У�![]() ��������Ԫ�صĻ��ϼ�Ϊ______________��
��������Ԫ�صĻ��ϼ�Ϊ______________��
��2�����е����������У��������������____________�������ʵ����ƣ���
��3��������Ӧ�Ļ�ѧ����ʽΪ______________��
��4��һλͬѧ�ڼ���x��y��z��ֵ�Ĺ����У��г������е�ʽ��������ȷ����_____________������ĸ��ţ���
Ax+y+z=169 By+z=168 C��100-x����z=32��64 D��l00-x������y-1��=8��3