��Ŀ����
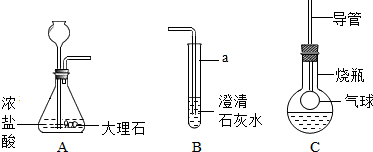
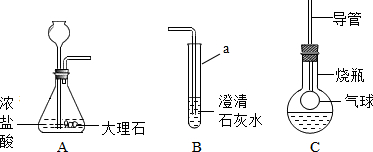
��ͼ��ij����С���ͬѧ��Ƶ�ʵ������ȡCO2�����������ʵ�װ��ʾ��ͼ
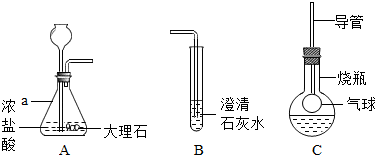
��ش���������
��1������a��ָ������������
��2����A�в���������ͨ�뵽B��һ�����δ�����б���ǣ�����ͬѧ��Ϊ����Ũ����ӷ�����HCl����������£�
���û�ѧ����ʽ��ʾΪ��Ca��OH��2+CO2=CaCO3��+H2O CaCO3+2HCl=CaCl2+H2O+CO2��
��Ľ��ͻ��ɸ���ݣ�����һ����ѧ����ʽ��ʾ��
��3���Ż�ͬѧ����ƿ�ռ�A�в���������������м��������ɫ����M��Һ���Cװ�ã���������ƿ�е������������Һʼ����ɫ������M������
��4�������һ����������֤Cװ���з�Ӧ���������к���CO32-��
��5����A��ҩƷ���ɹ���������Һ�Ͷ������̣���Ӧ�Ļ�ѧ����ʽΪ

��ش���������
��1������a��ָ������������
��ƿ
��ƿ
��Aװ���з����Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
������A�г���©�����ɷ�Һ©�����ŵ������Ʒ�Ӧ�ķ�����ֹͣ
���Ʒ�Ӧ�ķ�����ֹͣ
����2����A�в���������ͨ�뵽B��һ�����δ�����б���ǣ�����ͬѧ��Ϊ����Ũ����ӷ�����HCl����������£�
���û�ѧ����ʽ��ʾΪ��Ca��OH��2+CO2=CaCO3��+H2O CaCO3+2HCl=CaCl2+H2O+CO2��
��Ľ��ͻ��ɸ���ݣ�����һ����ѧ����ʽ��ʾ��
2HCl+Ca��OH��2=CaCl2+2H2O
2HCl+Ca��OH��2=CaCl2+2H2O
����3���Ż�ͬѧ����ƿ�ռ�A�в���������������м��������ɫ����M��Һ���Cװ�ã���������ƿ�е������������Һʼ����ɫ������M������
NaOH����KOH��
NaOH����KOH��
����дһ�����ʵĻ�ѧʽ������4�������һ����������֤Cװ���з�Ӧ���������к���CO32-��
| ���� | ���� | ���� |
| ȡ��Ӧ�����Һ�����������еμ� BaCl2��Һ��������ϡ���� BaCl2��Һ��������ϡ���� |
�а�ɫ�������ɻ�����ð�� �а�ɫ�������ɻ�����ð�� |
Cװ���з�Ӧ���������к���CO32- |
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
������ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ�����A�в���������ͨ�뵽B��һ�����δ�����б���ǣ�����ݵĽ����ǣ����������������Һ��Ӧ�����Ȼ��ƺ�ˮ����ƽ���ɣ���������ƿ�е������������Һʼ����ɫ����˵��������̼��M��Һ��Ӧ�ޱ仯��M��Һ��NaOH����KOH����Һ��Ҫ֤���������к���CO32-�������м���BaCl2��Һ��������ϡ���ᶼ�ɣ����������ڶ�������������������������ˮ����������ƽ���ɣ�
����⣺��1����ƿ�dz��õķ�Ӧ������̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ���Һ©�����Կ��Ʒ�Ӧ�ķ�����ֹͣ��
�ʴ�Ϊ����ƿ��CaCO3+2HCl=CaCl2+H2O+CO2�������Ʒ�Ӧ�ķ�����ֹͣ��
��2����A�в���������ͨ�뵽B��һ�����δ�����б���ǣ�����ݵĽ����ǣ����������������Һ��Ӧ�����Ȼ��ƺ�ˮ����ƽ���ɣ�
�ʴ�Ϊ��2HCl+Ca��OH��2=CaCl2+2H2O
��3����������ƿ�е������������Һʼ����ɫ����˵��������̼��M��Һ��Ӧ�ޱ仯��M��Һ��NaOH����KOH����Һ���ʴ�Ϊ��NaOH����KOH��
��4��Ҫ֤���������к���CO32-�������м���BaCl2��Һ��������ϡ���ᶼ�ɣ��������м���BaCl2��Һ�������ɰ�ɫ����������֤�����к�CO32-��
�ʴ�Ϊ��BaCl2��Һ��������ϡ����а�ɫ�������ɻ�����ð����
��5�����������ڶ�������������������������ˮ����������ƽ���ɣ��ʴ�Ϊ��2H2O2
2H2O+O2��
�ʴ�Ϊ����ƿ��CaCO3+2HCl=CaCl2+H2O+CO2�������Ʒ�Ӧ�ķ�����ֹͣ��
��2����A�в���������ͨ�뵽B��һ�����δ�����б���ǣ�����ݵĽ����ǣ����������������Һ��Ӧ�����Ȼ��ƺ�ˮ����ƽ���ɣ�
�ʴ�Ϊ��2HCl+Ca��OH��2=CaCl2+2H2O
��3����������ƿ�е������������Һʼ����ɫ����˵��������̼��M��Һ��Ӧ�ޱ仯��M��Һ��NaOH����KOH����Һ���ʴ�Ϊ��NaOH����KOH��
��4��Ҫ֤���������к���CO32-�������м���BaCl2��Һ��������ϡ���ᶼ�ɣ��������м���BaCl2��Һ�������ɰ�ɫ����������֤�����к�CO32-��
�ʴ�Ϊ��BaCl2��Һ��������ϡ����а�ɫ�������ɻ�����ð����
��5�����������ڶ�������������������������ˮ����������ƽ���ɣ��ʴ�Ϊ��2H2O2
| ||
��������������Ҫ�����˶�����̼���������Ʒ���̼������ӵļ��飬ͬʱҲ�����˶�����̼�����ʺͻ�ѧ����ʽ����д���ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

��ϰ��ϵ�д�
�����Ŀ