��Ŀ����
ȼ�շ�Ӧ�������ƶ�������Ľ�����ȼ�������ǵ����������ķ�չ�������е���ϵ��
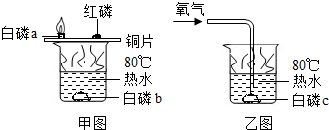
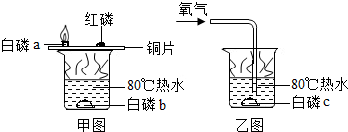
��1������ȼ������̽����ʵ��
����ͼ�����Ļ�ѧ����ʽΪ
�ڸ���
��2��ȼ��������
��ȼ������ᷢչ������������Ҫ�����ã�����Ӧ����������Դ�ͱ���������
Ŀǰ����ͨ����ѧ��Ӧ��õ��������������
��ȼú����ʱ����ú������ú�ۣ���Ŀ����
��Ŀǰ��������Դ��
���Ҵ�����
�ݻ�ѧ�仯ͨ�����������ı仯����д��һ��Ϊ���ȷ�Ӧ�ķ���ʽ
��3��ȼ�������
������ȼ�������Σ����������վ�Ż𣬿�����

��1������ȼ������̽����ʵ��
����ͼ�����Ļ�ѧ����ʽΪ
4P+5O2
2P2O5
| ||
4P+5O2
2P2O5
| ||
�ڸ���
ͭƬ�ϵİ���ȼ�գ�ˮ�а��ײ�����
ͭƬ�ϵİ���ȼ�գ�ˮ�а��ײ�����
�����ܵó�ȼ����Ҫ���������������Ľ��ۣ���2��ȼ��������
��ȼ������ᷢչ������������Ҫ�����ã�����Ӧ����������Դ�ͱ���������
Ŀǰ����ͨ����ѧ��Ӧ��õ��������������
ú
ú
��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ���Ȼ������Ҫ�ɷ�Ϊ���ѧʽ��CH4
CH4
����ȼ�յĻ�ѧ����ʽΪCH4+2O2
CO2+2H2O
| ||
CH4+2O2
CO2+2H2O
����Ϊ��ʯȼ����Դ���ޣ������������úͿ�����ϫ�ܡ������ܡ�
| ||
̫����
̫����
������Դ����ȼú����ʱ����ú������ú�ۣ���Ŀ����
����Ӧ�ĽӴ����
����Ӧ�ĽӴ����
����Ŀǰ��������Դ��
����
����
��û�д���ʹ�õ�ԭ�����������ѣ���ȡ�ɱ���
�������ѣ���ȡ�ɱ���
�����Ҵ�����
������
������
��Դ����������������������������ȼ�յķ���ʽΪC2H5OH+3O2
2CO2+3H2O
| ||
C2H5OH+3O2
2CO2+3H2O
| ||
�ݻ�ѧ�仯ͨ�����������ı仯����д��һ��Ϊ���ȷ�Ӧ�ķ���ʽ
CO2+C
2CO
| ||
CO2+C
2CO
| ||
��3��ȼ�������
������ȼ�������Σ����������վ�Ż𣬿�����
�ɷ�
�ɷ�
�������ͼ����Ż������Һ̬������̼
Һ̬������̼
���������������1���ٽ�����ͨ�������ˮ�У�����Ҫ��������Ӧ������д�����Ƿ�Ӧ�Ļ�ѧ����ʽ�����Ծݴ˽��
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ���������Ծݴ˽��
��2����ú��ʯ�͡���Ȼ��Ϊ����ʯȼ���Լ�����ȼ�����ɶ�����̼��ˮ���н��
�ڸ���ȼú����ʱ����ú������ú�ۣ���Ŀ��������Ӧ�ĽӴ�������н��
�۸�������ȼ������ˮ��������������Դ��û�д���ʹ�õ�ԭ�����������ѣ���ȡ�ɱ��߽��н��
�ܸ����Ҵ����ڿ�������Դ�Լ�ȼ�����ɶ�����̼��ˮ���н��
�ݸ��ݶ�����̼��̼�ڸ�������������һ����̼�������ȷ�Ӧ���н��
��3������ȼ�յ�������ѡ������ԭ����
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ���������Ծݴ˽��
��2����ú��ʯ�͡���Ȼ��Ϊ����ʯȼ���Լ�����ȼ�����ɶ�����̼��ˮ���н��
�ڸ���ȼú����ʱ����ú������ú�ۣ���Ŀ��������Ӧ�ĽӴ�������н��
�۸�������ȼ������ˮ��������������Դ��û�д���ʹ�õ�ԭ�����������ѣ���ȡ�ɱ��߽��н��
�ܸ����Ҵ����ڿ�������Դ�Լ�ȼ�����ɶ�����̼��ˮ���н��
�ݸ��ݶ�����̼��̼�ڸ�������������һ����̼�������ȷ�Ӧ���н��
��3������ȼ�յ�������ѡ������ԭ����
����⣺��1���ٽ�����ͨ�������ˮ�У�����Ҫ��������Ӧ�������������ף����Ƿ�Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
2P2O5��
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ������
��2����ú��ʯ�͡���Ȼ��Ϊ����ʯȼ�ϣ���Ȼ������Ҫ�ɷ�Ϊ���飬����ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2
CO2+2H2O����Ϊ��ʯȼ����Դ���ޣ������������úͿ�����ϫ�ܡ������ܡ�̫���ܵ�����Դ��
��ȼú����ʱ����ú������ú�ۣ���Ŀ��������Ӧ�ĽӴ������
������ȼ������ˮ��������������Դ��û�д���ʹ�õ�ԭ�����������ѣ���ȡ�ɱ��ߣ�
���Ҵ����ڿ�������Դ��ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
2CO2+3H2O��
�ݶ�����̼��̼�ڸ�������������һ����̼�������ȷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CO2+C
2CO��
��3��������ȼ�������Σ����������վ�Ż𣬿����øɷ��������ͼ����Ż������Һ̬������̼�������
�ʴ�Ϊ����1����4P+5O2
2P2O5��
��ͭƬ�ϵİ���ȼ�գ�ˮ�а��ײ����գ�
��2����ú��CH4��̫���ܵȣ�
������Ӧ�ĽӴ������
���������������ѣ���ȡ�ɱ��ߣ�
�ܿ�������C2H5OH+3O2
2CO2+3H2O�� ��CO2+C
2CO��
��3���ɷۣ� Һ̬������̼��
| ||
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ������
��2����ú��ʯ�͡���Ȼ��Ϊ����ʯȼ�ϣ���Ȼ������Ҫ�ɷ�Ϊ���飬����ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2
| ||
��ȼú����ʱ����ú������ú�ۣ���Ŀ��������Ӧ�ĽӴ������
������ȼ������ˮ��������������Դ��û�д���ʹ�õ�ԭ�����������ѣ���ȡ�ɱ��ߣ�
���Ҵ����ڿ�������Դ��ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
| ||
�ݶ�����̼��̼�ڸ�������������һ����̼�������ȷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CO2+C
| ||
��3��������ȼ�������Σ����������վ�Ż𣬿����øɷ��������ͼ����Ż������Һ̬������̼�������
�ʴ�Ϊ����1����4P+5O2
| ||
��ͭƬ�ϵİ���ȼ�գ�ˮ�а��ײ����գ�
��2����ú��CH4��̫���ܵȣ�
������Ӧ�ĽӴ������
���������������ѣ���ȡ�ɱ��ߣ�
�ܿ�������C2H5OH+3O2
| ||
| ||
��3���ɷۣ� Һ̬������̼��
��������������Ŀʱ�����Ը���ȼ�յ�������ѧ�������֪ʶ������ھ�ʵ��ͼʾ�е�������Ϣ��ʹ�ÿ��Ʊ�����������ʵ�鷨�������ʵ�鷽�������߸��������龰�����ո�����ʵ�鷽�������п�ѧʵ��̽����Ȼ�������ɳ�ȼ�յ����������ԭ���ȣ��������ʵ�����ã���һ����չ��Ǩ�ƣ��Ա���������ʵ�����⣮

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
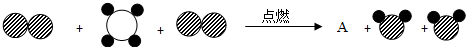
 ����ʾ̼ԭ�ӣ���
����ʾ̼ԭ�ӣ��� ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ
����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ

 ����ʾ̼ԭ�ӣ���
����ʾ̼ԭ�ӣ��� ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ
����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ


 ����ʾ̼ԭ�ӣ���
����ʾ̼ԭ�ӣ��� ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ ������ĸ��ţ���
����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ ������ĸ��ţ���


 ����ʾ̼ԭ�ӣ���
����ʾ̼ԭ�ӣ��� ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ______������ĸ��ţ���
����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ______������ĸ��ţ���