��Ŀ����
��2012?�ߴ���һģ��ȼ�շ�Ӧ�������ƶ�������Ľ�����ȼ�������ǵ����������ķ�չ�������е���ϵ��
��1������ȼ������̽����ʵ�飺
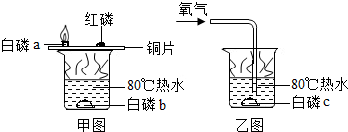
�ټ�ͼͭƬ�ϵİ�����ȼ�գ����ײ���ȼ�գ��ɴ������ܵó��Ľ�����
�ڸ���
����ͼ�з�����Ӧ�Ļ�ѧ��ʽ��Ϊ
��2��ȼ��������
��ȼ�ղ�������Ϊ�������ã���ʯȼ���ǵ�ǰ�������Ҫȼ�ϣ�����ú��
CO+H2���÷�Ӧ�б��ֳ���ԭ�Ե�������
��ij��Դ����ȼ�յ��۹������£�
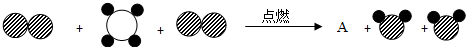
���� ����ʾ̼ԭ�ӣ���
����ʾ̼ԭ�ӣ��� ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ
����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ
�۾ƾ�����ʵ���ҵij�����Դ��д���ƾ�ȼ�յĻ�ѧ��ʽ��Ϊ
��������Ҫ��̿����ԭ�ϣ����������н�̿������������
��3��ȼ�������
������ȼ�������Σ����ȼ�ջ�������ը���ƻ�ȼ������������𣬱��ⱬը����ͥ����ʱ���Ż���ù��Ǹ�������ԭ����
��4������ȼ�ս��п�ѧʵ��
��Ϊ̽����˿�Ĵ�ϸ�̶ȶ�����������ȼ�յ�Ӱ�죬����ʵ���ܴﵽĿ����
A����ͬһƿ�����У��Ⱥ���д֡�ϸ��˿��ȼ��ʵ��
B������ƿ��ͬŨ�ȵ������У�ͬʱ���д֡�ϸ��˿��ȼ��ʵ��
C������ƿ��ͬŨ�ȵ������У�ͬʱ���д֡�ϸ��˿��ȼ��ʵ��
D������ƿ��ͬŨ�ȵ������У�ͬʱ������ͬ��˿��ȼ��ʵ��
��3.2gij���ʵ���ȫȼ�ղ���4.4gCO2��3.6gH2O��û���ɱ�����ʣ���������C��HԪ��������Ϊ
��1������ȼ������̽����ʵ�飺

�ټ�ͼͭƬ�ϵİ�����ȼ�գ����ײ���ȼ�գ��ɴ������ܵó��Ľ�����
����������Ż�㲻ͬ
����������Ż�㲻ͬ
���ڸ���
��ͼˮ�а��ײ�ȼ�գ�ͭƬ�ϰ���ȼ��
��ͼˮ�а��ײ�ȼ�գ�ͭƬ�ϰ���ȼ��
�����ܵó�ȼ����Ҫ�����Ľ��ۣ�����ͼ�з�����Ӧ�Ļ�ѧ��ʽ��Ϊ
4P+5O2
2P2O5
| ||
4P+5O2
2P2O5
��
| ||
��2��ȼ��������
��ȼ�ղ�������Ϊ�������ã���ʯȼ���ǵ�ǰ�������Ҫȼ�ϣ�����ú��
ʯ��
ʯ��
����Ȼ����ֱ����ú������ȼ�ϼ���Ⱦ�������˷���Դ��ú�������ǰ�ú��Ϊ�����Դ����Ҫһ��������һ����Ӧ�ǣ�C+H2O
| ||
C
C
����ij��Դ����ȼ�յ��۹������£�
����
 ����ʾ̼ԭ�ӣ���
����ʾ̼ԭ�ӣ���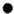 ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪ
����ʾ��ԭ�ӣ��÷�Ӧ��������AӦΪb
b
������ĸ��ţ���a��CO b��CO2�۾ƾ�����ʵ���ҵij�����Դ��д���ƾ�ȼ�յĻ�ѧ��ʽ��Ϊ
C2H5OH+3O2
2CO2+3H2O
| ||
C2H5OH+3O2
2CO2+3H2O
��
| ||
��������Ҫ��̿����ԭ�ϣ����������н�̿������������
ȼ���ṩ������ά�ַ�Ӧ�����¶Ȳ�����CO
ȼ���ṩ������ά�ַ�Ӧ�����¶Ȳ�����CO
���ô�������Ҫ�ɷ�ΪFe3O4�������Ļ�ѧ��ʽ��ΪFe3O4+4CO
3Fe+4CO2
| ||
Fe3O4+4CO
3Fe+4CO2
��
| ||
��3��ȼ�������
������ȼ�������Σ����ȼ�ջ�������ը���ƻ�ȼ������������𣬱��ⱬը����ͥ����ʱ���Ż���ù��Ǹ�������ԭ����
������������������
������������������
��CO2��������һ���ȼ������Ļ��֣���������������ȼ���Ҳ���ȼ
����ȼ���Ҳ���ȼ
���ܶȱȿ���������ʣ���4������ȼ�ս��п�ѧʵ��
��Ϊ̽����˿�Ĵ�ϸ�̶ȶ�����������ȼ�յ�Ӱ�죬����ʵ���ܴﵽĿ����
C
C
��A����ͬһƿ�����У��Ⱥ���д֡�ϸ��˿��ȼ��ʵ��
B������ƿ��ͬŨ�ȵ������У�ͬʱ���д֡�ϸ��˿��ȼ��ʵ��
C������ƿ��ͬŨ�ȵ������У�ͬʱ���д֡�ϸ��˿��ȼ��ʵ��
D������ƿ��ͬŨ�ȵ������У�ͬʱ������ͬ��˿��ȼ��ʵ��
��3.2gij���ʵ���ȫȼ�ղ���4.4gCO2��3.6gH2O��û���ɱ�����ʣ���������C��HԪ��������Ϊ
3��1
3��1
������������
��
�����������������Ԫ�أ���������1���ٺ��Ͱ�������ͬ�Ļ����У�������ȼ�ն����ײ���ȼ�գ�˵������ȼ��������¶Ȳ�ͬ�����Ծݴ˽��
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ���������Ծݴ˽��
�۽�����ͨ�������ˮ�У�����Ҫ��������Ӧ������д�����Ƿ�Ӧ�Ļ�ѧ����ʽ��
��2����úʯ����Ȼ��Ϊ����ʯȼ�ϣ���������ԭ��Ӧ�е�����Ϊ��ԭ�������Ծݴ˽��
�ڸ��ݷ�Ӧǰ��ԭ�ӵ����������������ɽ��
�۾ƾ�ȼ�ղ����˶�����̼��ˮ�����Ծݴ�д���÷�Ӧ�Ļ�ѧ����ʽ��
�ܸ��ݽ�̿�����������н�𣬲�д��һ����̼�ʹ������������������ķ�Ӧ�Ļ�ѧ����ʽ��
��3������ȼ�յ�������ѡ������ԭ����
��4�������ÿ��Ʊ�����������̽��ʵ�飻
�ڸ��������غ㶨�ɿ���֪����Ӧǰ��Ԫ�ص�����������䣬���Ծݴ������
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ���������Ծݴ˽��
�۽�����ͨ�������ˮ�У�����Ҫ��������Ӧ������д�����Ƿ�Ӧ�Ļ�ѧ����ʽ��
��2����úʯ����Ȼ��Ϊ����ʯȼ�ϣ���������ԭ��Ӧ�е�����Ϊ��ԭ�������Ծݴ˽��
�ڸ��ݷ�Ӧǰ��ԭ�ӵ����������������ɽ��
�۾ƾ�ȼ�ղ����˶�����̼��ˮ�����Ծݴ�д���÷�Ӧ�Ļ�ѧ����ʽ��
�ܸ��ݽ�̿�����������н�𣬲�д��һ����̼�ʹ������������������ķ�Ӧ�Ļ�ѧ����ʽ��
��3������ȼ�յ�������ѡ������ԭ����
��4�������ÿ��Ʊ�����������̽��ʵ�飻
�ڸ��������غ㶨�ɿ���֪����Ӧǰ��Ԫ�ص�����������䣬���Ծݴ������
����⣺��1���ٺ��Ͱ�������ͬ�Ļ����У�������ȼ�ն����ײ���ȼ�գ�˵������ȼ��������¶Ȳ�ͬ��
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ������
�۽�����ͨ�������ˮ�У�����Ҫ��������Ӧ�������������ף����Ƿ�Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
2P2O5��
��2����úʯ����Ȼ��Ϊ����ʯȼ�ϣ�̼�ڷ�Ӧ�е�����Ϊ��ԭ���������л�ԭ�Ե�����Ϊ̼��
�ڸ��ݷ�Ӧǰ��ԭ�ӵ���������������֪��A�ķ����к���������ԭ�Ӻ�һ��̼ԭ�ӣ�����AΪ������̼����ѡb��
�۾ƾ�ȼ�ղ����˶�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
3H2O+2CO2��
�ܽ�̿����ȼ�շų������������������Ĺ������ṩ������ͬʱ��Ӧ�ܹ����ɻ�ԭ��һ����̼����ԭ�������������е���������һ����̼�ʹ������������������ķ�Ӧ�Ļ�ѧ����ʽΪ��Fe3O4+4CO
3Fe+4CO2��
��3����ͥ����ʱ���Ż���ù��Ǹ�������ԭ���Ǹ�����������CO2��������һ���ȼ������Ļ��֣����������IJ���֧��ȼ�պ��ܶȱȿ���������ʣ�
��4����Ϊ̽����˿�Ĵ�ϸ�̶ȶ�����������ȼ�յ�Ӱ�죬���ÿ��Ʊ���������֪��Ӧ�ñ�֤������Ũ����ͬ�������²�ȡһ��һϸ����˿������ͬʱ��ȼ������������ѡ�����֪��ѡ��C����ȷ�ģ���ѡC��
�ڸ��������غ㶨�ɿ���֪����Ӧǰ��Ԫ�ص���������������Ը����ʺ��е�̼Ԫ�غͶ�����̼�к��е�̼Ԫ�ص�������ȣ������ʺ��е���Ԫ�غ�ˮ����Ԫ�ص�������ȣ�������̼��̼Ԫ�ص�����Ϊ��4.4g��
��100%=1.2g��ˮ����Ԫ�ص�����Ϊ��3.6g��
��100%=0.4g������������C��HԪ��������Ϊ1.2g��0.4g=3��1��1.2g+0.4g=1.6g��3.2g�������ڸ������л�������Ԫ�أ�
�ʴ�Ϊ��
��1���ٺ���������Ż�㲻ͬ���ڼ�ͼˮ�а��ײ�ȼ�գ�ͭƬ�ϰ���ȼ�գ���4P+5O2
2P2O5��
��2����ʯ�ͣ�C����b����C2H5OH+3O2
2CO2+3H2O��
��ȼ���ṩ������ά�ַ�Ӧ�����¶Ȳ�����CO��Fe3O4+4CO
3Fe+4CO2��
��3��������������������������ȼ���Ҳ���ȼ��
��4����C����3��1������
�ڿ����еİ�����ȼ�գ���ˮ�еİ��ײ���ȼ�գ�˵��ȼ����Ҫ������
�۽�����ͨ�������ˮ�У�����Ҫ��������Ӧ�������������ף����Ƿ�Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
| ||
��2����úʯ����Ȼ��Ϊ����ʯȼ�ϣ�̼�ڷ�Ӧ�е�����Ϊ��ԭ���������л�ԭ�Ե�����Ϊ̼��
�ڸ��ݷ�Ӧǰ��ԭ�ӵ���������������֪��A�ķ����к���������ԭ�Ӻ�һ��̼ԭ�ӣ�����AΪ������̼����ѡb��
�۾ƾ�ȼ�ղ����˶�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
| ||
�ܽ�̿����ȼ�շų������������������Ĺ������ṩ������ͬʱ��Ӧ�ܹ����ɻ�ԭ��һ����̼����ԭ�������������е���������һ����̼�ʹ������������������ķ�Ӧ�Ļ�ѧ����ʽΪ��Fe3O4+4CO
| ||
��3����ͥ����ʱ���Ż���ù��Ǹ�������ԭ���Ǹ�����������CO2��������һ���ȼ������Ļ��֣����������IJ���֧��ȼ�պ��ܶȱȿ���������ʣ�
��4����Ϊ̽����˿�Ĵ�ϸ�̶ȶ�����������ȼ�յ�Ӱ�죬���ÿ��Ʊ���������֪��Ӧ�ñ�֤������Ũ����ͬ�������²�ȡһ��һϸ����˿������ͬʱ��ȼ������������ѡ�����֪��ѡ��C����ȷ�ģ���ѡC��
�ڸ��������غ㶨�ɿ���֪����Ӧǰ��Ԫ�ص���������������Ը����ʺ��е�̼Ԫ�غͶ�����̼�к��е�̼Ԫ�ص�������ȣ������ʺ��е���Ԫ�غ�ˮ����Ԫ�ص�������ȣ�������̼��̼Ԫ�ص�����Ϊ��4.4g��
| 12 |
| 12+16��2 |
| 1��2 |
| 1��2+16 |
�ʴ�Ϊ��
��1���ٺ���������Ż�㲻ͬ���ڼ�ͼˮ�а��ײ�ȼ�գ�ͭƬ�ϰ���ȼ�գ���4P+5O2
| ||
��2����ʯ�ͣ�C����b����C2H5OH+3O2
| ||
��ȼ���ṩ������ά�ַ�Ӧ�����¶Ȳ�����CO��Fe3O4+4CO
| ||
��3��������������������������ȼ���Ҳ���ȼ��
��4����C����3��1������
���������������Ŀʱ�����Ը���ȼ�յ�������ѧ�������֪ʶ������ھ�ʵ��ͼʾ�е�������Ϣ��ʹ�ÿ��Ʊ�����������ʵ�鷨�������ʵ�鷽�������߸��������龰�����ո�����ʵ�鷽�������п�ѧʵ��̽����Ȼ�������ɳ�ȼ�յ����������ԭ���ȣ��������ʵ�����ã���һ����չ��Ǩ�ƣ��Ա���������ʵ�����⣮

��ϰ��ϵ�д�
�����Ŀ