��Ŀ����
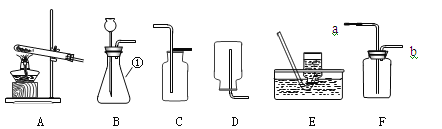
����Ŀ��������������ʯ�ң�ijͬѧ������������������ʵ�鷽����
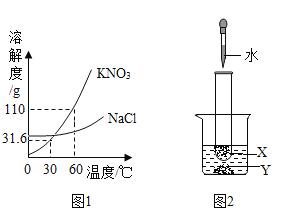
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ_____������B��������IJ������_____
��2�����Ҫ��ʵ��֤��C�в���ˮ����һ����Һ������ѡ������_____������ţ�����ʵ�顣
��ϡ���� �ڷ�̪��Һ �۶�����̼ ���Ȼ�����Һ
��3���������Ǽ���ij������ʹ��ɫ����Һ���ְ�ɫ���壬���������DZ���̼������Һ����ɫ����Ļ�ѧʽ��_____�����������ǹ����������ƣ�ȫ���ܽ⣩����ɫ����Ļ�ѧʽ��_____��
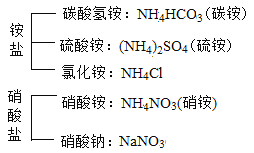
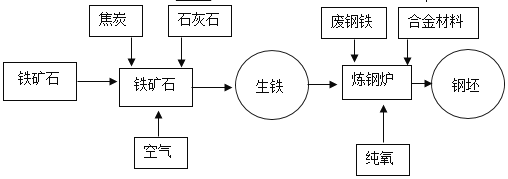
���������ϣ���ҵ������Ҫ�ɷ���Fe2O3��������������FeO��Fe3O4
��1�����ᾧ�壨H2C2O4����3H2O����Ũ�������������ȷֽ⣬��ѧ����ʽ_____��
��2����ʯ���ǹ���NaOH��CaO�Ļ���������ˮ�����Ͷ�����̼��
��3�����ij�������������������������
���������� | FeO | Fe2O3 | Fe3O4 |
������������ | 77.8% | 70.0% | 72.4% |
���������ۣ�Ϊ�˲ⶨ��������������������С��ͬѧ��������ʵ�顣��װ�����������ã�
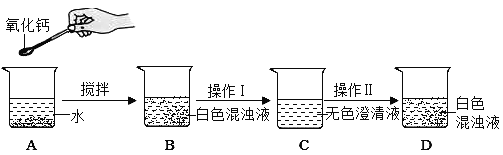
��1����ʵ��Ϊ�˱�֤����E�е������Ǵ����������CO����B��C��D�е��Լ�������_____������ĸ��ţ���
a.Ũ���� b.�����ʯ��ˮ c.����������Һ
��2��Cװ�õ�������_____��
��3��д��Eװ������������Ӧ��һ����ѧ����ʽ��_____��
��4����ȡ������Ʒ10.0g��������װ�ý���ʵ�飬�ⶨ��������������������
����E�г�ַ�Ӧ��õ����۵�����Ϊm g����_____<m��_____��
����ʵ��ǰ��Ƶ�Fװ������7.7g�������������������������_____
��ʵ�鷴˼��
��1�����ȱ��Gװ�ã��������������أ���������Ʒ����������������_____��ѡ�ƫС�������䡱��ƫ����
��2����ʵ��װ�õ�һ������ȱ����_____��
���𰸡�CaO+H2O�TCa��OH��2 ���� �ڢ� CaCO3 Ca��OH��2 H2C2O43H2O CO2��+CO��+4H2O cba ���������̼�����Ƿ���� 3CO+Fe2O3
CO2��+CO��+4H2O cba ���������̼�����Ƿ���� 3CO+Fe2O3![]() 2Fe+3CO2����4CO+Fe3O4
2Fe+3CO2����4CO+Fe3O4![]() 3Fe+4CO2��CO+FeO
3Fe+4CO2��CO+FeO![]() Fe+CO2�� 7.0g 7.78g 72% ƫС ȱ��β������װ��
Fe+CO2�� 7.0g 7.78g 72% ƫС ȱ��β������װ��
��������
��1����������ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽ��CaO+H2O�TCa��OH��2�����˿��Խ���������룬���Է���B��������IJ�����й��ˣ�
��2����̪������ɫ��������̼���������ƻ�����̼��Ƴ�����������������Ʒ�Ӧû�����������Ȼ��Ʋ������������Ʒ�Ӧ����ѡ�ڢۣ�
��3��̼�������������Ʒ�Ӧ����̼��ư�ɫ�������������ƣ���ɫ����Ļ�ѧʽ��CaCO3��������������ˮ���ȣ��������Ƶ��ܽ�����¶ȵ����߶���С�����Լ�����ǹ����������ƣ�ȫ���ܽ⣩����ɫ����Ļ�ѧʽ��Ca��OH��2��
[��������]��1�����ᾧ�壨H2C2O43H2O����Ũ�������������ȷֽ⣬��ѧ����ʽΪ��H2C2O43H2O CO2��+CO��+4H2O��
CO2��+CO��+4H2O��
[��������]��1����ȡ�������к���һ����̼��������̼��ˮ������Ҫ�õ������������CO������Ҫ��������������Һ��ȥ���еĶ�����̼��Ȼ���ó����ʯ��ˮ�����������̼�Ƿ���ȫ��ȥ�������Ũ������и��
��2��������̼��ʹ�����ʯ��ˮ����ǣ�����װ��C�������Ǽ��������̼�����Ƿ������
��3���ڸ��µ������£�һ����̼�ܽ����������ﻹԭΪ����ͬʱ���ɶ�����̼����ѧ����ʽΪ��3CO+Fe2O3![]() 2Fe+3CO2����4CO+Fe3O4
2Fe+3CO2����4CO+Fe3O4![]() 3Fe+4CO2��CO+FeO
3Fe+4CO2��CO+FeO![]() Fe+CO2����
Fe+CO2����
��4���ٸ�����������������������ĺ�������֪������������Ԫ�ص�����������С��������������Ԫ�ص�������������������������ȫΪ����������E�г�ַ�Ӧ��õ����۵�����Ϊ10g��70%=7.0g��������������ȫΪ������������E�г�ַ�Ӧ��õ����۵�����Ϊ10g��77.8%=7.78g����ҵ������Ҫ�ɷ���Fe2O3��������������FeO��Fe3O4����E�г�ַ�Ӧ��õ����۵���������7.0g��7.78g֮�䣻
��װ��F���ص�������Ϊһ����̼��ԭ��������������ɵĶ�����̼���������ɻ�ѧ����ʽ�Լ������غ㶨�ɿ�֪��������̼�е���Ԫ����һ���������������������Ϊ��7.7g��![]() ��100%��
��100%��![]() =2.8g�������������������������
=2.8g�������������������������![]() ��100%=72%��
��100%=72%��
[ʵ�鷴˼]��1����û��װ��G����F�����տ����е�ˮ�����Ͷ�����̼������ƫ������������Ԫ������ƫ���²����Ʒ��������������ƫС��
��2��һ����̼�ж����ŷŵ������л���Ⱦ����������ʵ��װ�õ�һ������ȱ�ݣ�ȱ��β������װ�á�

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ��![]()
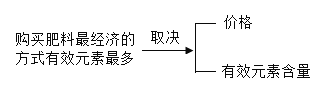
�������� | ��ֲ������� | |
���� -N | ���أ�CO(NH2)2��N%��46.7%����Ч�ʣ� ��ˮ����NH3��H2O��
| |
�� -P | ��ۣ�Ca3(PO4)2 ��þ�ʣ��ƺ�þ��������
| |
�ط� -K | ����أ�K2SO4 �Ȼ��أ�KCl | |
���� ���� |
| ���ԣ� |
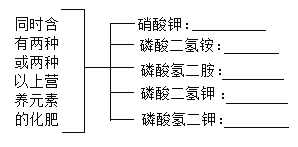
�����пո���������________�������________����������________����������________����������________�����������________