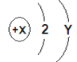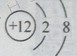��Ŀ����
����Ŀ������������������Ӧ�úܹ㷺��һ�ֽ����������������������йصIJ���ʵ��ͼ����ش��������⡣
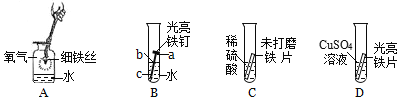
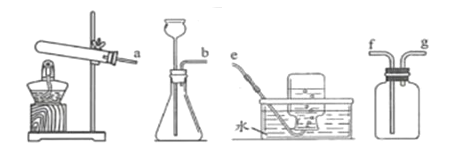
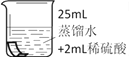
��1��A��ϸ��˿ȼ�����ɺ�ɫ�������ʵĻ�ѧʽ��________��
��2��B��������������IJ��_______���a������b����c������
��3��C�иտ�ʼ�����ݲ�������һ���ֲ������ݣ�д���������巴Ӧ�Ļ�ѧ����ʽ��_______��
��4��D�з�Ӧһ��ʱ����Թ��ڹ��������ȷ�Ӧǰ���ݴ��ƶϣ��Թ�����Һ�����뷴Ӧǰ���________������������䡱��С������
���𰸡�Fe3O4 b Fe+H2SO4�TFeSO4+H2�� ��С
��������
��1��A��ϸ��˿ȼ�����ɺ�ɫ������������������������ѧʽΪ��Fe3O4��
��2��B������b����ˮ������ͬʱ�Ӵ����������⣬���b��
��3��C�иտ�ʼ�����ݲ�����������δ��ĥ��Ƭ����������⣬���������ⷴӦ����һ���ֲ��������������������ᷴӦ���������������������������巴Ӧ�Ļ�ѧ����ʽ��Fe+H2SO4�TFeSO4+H2����
��4��D����������ͭ��Ӧ��������������ͭ�����ݻ�ѧ����ʽFe+CuSO4=Cu+FeSO4��֪��ÿ56�������������û���64��������ͭ���ʷ�Ӧһ��ʱ����Թ�����Һ�����뷴Ӧǰ��ȼ�С��

����Ŀ����һ�������£���һ���ܱ������ڷ���ij��Ӧ����÷�Ӧ�����и����ʵ����������ʾ������˵������ȷ���ǣ�������
���� | X | Y | Z | W |
��Ӧǰ����/g | 10 | 3 | 90 | 0 |
��Ӧ������/g | 3.2 | 3 | ���� | 3.2 |
A. W�����ǵ��� B. Y�����Ǵ���
C. �÷�Ӧ�ǷֽⷴӦ D. ��Ӧ��Z���ʵ�����Ϊ![]()
����Ŀ�����������������������й㷺Ӧ�á�ʵ��С��Թ��������ijЩ���ʽ����о���
I�����ȶ���
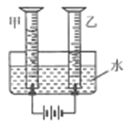
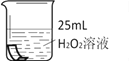
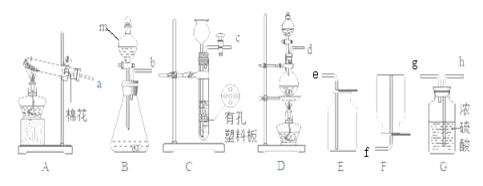
��1������ͼ��ʾ����ʵ�飬��������ֽ�Ļ�ѧ����ʽΪ_______________������3.2g O2ʱ�ֽ�Ĺ������������Ϊ______ g��

��2��������ˮ���ռ�O2��ԭ����______��
��3��̽���¶ȶԹ�������ֽ����ʵ�Ӱ��
ͬѧ�ǽ��������µ�ʵ�飬ʵ���������±���
ʵ����� | �� | �� | �� |
H2O2��Һ��Ũ�ȣ� | 30 | 30 | 30 |
H2O2��Һ�����/mL | 6 | 6 | 6 |
�¶�/�� | 20 | 35 | 55 |
MnO2������/g | 0 | 0 | 0 |
�ռ�O2�����/mL | 0 | 1.9 | 7.8 |
��Ӧʱ�� | 40min | 40min | 40 min |
�ɴ˵ó��Ľ�����_____________________________��
������ʴ��
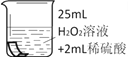
���������ϡ�H2O2��Һ�и�ʴ�ԡ�
������ʵ�顿
��ͭƬ�ֱ������3����Һ�н���ʵ�飬���±���
��� | �� | �� | �� |
ʵ�� |
|
|
|
һ��ʱ�������� | �����Ա仯 | ��Һ��������������ϸС���� | �����Ա仯 |
����������ۡ�
��4��ʵ��ٵ�������_____________________��
��5��ͭƬ����ʴ�ķ�Ӧ���£���ȫ�÷�Ӧ�Ļ�ѧ����ʽ��
Cu + H2O2+ H2SO4=== CuSO4 +_______��
����˼������
��6��ijͬѧ�����ʵ����У���������5���ķ�Ӧ�⣬��������һ����Ӧ������ϸС���ݲ������÷�Ӧ�ķ�Ӧ��Ϊ_______��
����Ŀ��ijͬѧ���̵���һ�֡�����������ͼ��������ԡ�����������̽����

��������⣩�����������Ƿ���̼���⡢��Ԫ�ء�
���������ϣ�������������ȫȼ�գ�ȼ��ʱ���̳���ȼ�չ��̺�Ϩ��ʱ����ζ��
��̽��ʵ�飩�����������ʵ�鱨�棩
���� | ���� | ���� |
��1����ȼ�������������ڻ����Ϸ���һ�������С�ձ��� | �ձ��ڱ�����ɫСҺ�β����� | ������������ȼ�պ�IJ�����________�� |
��2��Ѹ�ٵ�תС�ձ��������м�������__________���� | _____ | ������������ȼ�պ�IJ����ж�����̼�� |
��ʵ����ۣ���ͬѧˮ������Ԫ�غ���Ԫ����ɣ�������̼����̼Ԫ�غ���Ԫ����ɣ���ˡ�����������̼���⡢��Ԫ�ء����������жϵ�����������_______��
����˼�����ۣ�����Ϊ��ͬѧ�ó���ʵ������Ƿ���ȷ��_____�����ȷ������ȷ��������˵��ԭ��___________��