��Ŀ����
ij��ҵ��һ���������������ƷΪ���ĵط�������ҵ���ڵ������������У����ɱ���ػ����һ�����ĺ�ͭ���ϣ��磺������£���ij��ѧ��ȤС���ͬѧ��֪��һ�������������ú�ͭ�����Ʊ�������������ͭ���Ʊ�
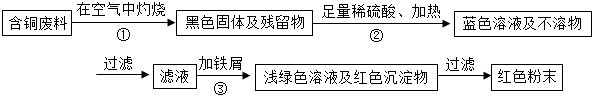
����1����ͬѧ������ѧ֪ʶ��������Ʊ�����ͭ������

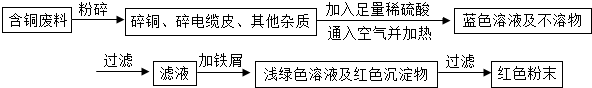
����2����ͬѧ���ݲ��������ҵ���һ�ֹ�ҵ�Ʊ�����ͭ�����̣�

��1�����������еĢ١��ڡ�����������ͭ��ͭ�Ļ������йصĻ�ѧ��Ӧ����ʽ�ֱ��ǣ�
��______ 2CuO

���𰸡����������Ӻ�ͭ�����л���ͭ��������ɫ��ѧ������ٻ��������У�������������������й����⣬���������ۣ������У��������йػ�ѧ����ʽΪ����2Cu+O2 2CuO����CuO+H2SO4�TCuSO4+H2O����Fe+CuSO4�TFeSO4+Cu���������ա��������Ⱦ���������塢�۳������������Բ����ҷ�����������Ȼ�������ȡ���й����⣻���ѧ���ļ���������������ճɹ�����ǿ�ɾУ�
2CuO����CuO+H2SO4�TCuSO4+H2O����Fe+CuSO4�TFeSO4+Cu���������ա��������Ⱦ���������塢�۳������������Բ����ҷ�����������Ȼ�������ȡ���й����⣻���ѧ���ļ���������������ճɹ�����ǿ�ɾУ�
����⣺��1��ͭ�ڿ������������ɺ�ɫ��������ͭ������ͭ�����ᷴӦ������ɫ����ͭ������м��Ӧ�û���ͭ����Ӧ����ʽΪ��2Cu+O2 2CuO��
2CuO��
CuO+H2SO4�TCuSO4+H2O�� Fe+CuSO4�TFeSO4+Cu��
��2���ӻ����Ƕȶ��������IJ�ͬ���ֽ��бȽϣ�������һ�����ջ������Ⱦ���������壬�۳����������ɴ˿�֪����2��������
��3��H2O2�����ֽ⣬�¶�Ӧ�ÿ�����50�浽60��֮�䣻
��4����ϡ���������Ϊx����36.8%x=1000mL×1.84g/mL×98%����x=4900g����Ҫˮ���������4900g-1000mL×1.84g/mL��÷1.0g/mL=3060mL����3.06L��
ϡ��Ũ����ķ����ǣ���Ũ�������ձ��ڻ�������ˮ�У����ò��������Ͻ��裮
��5������ͭ����������ˮ��������95%�ľƾ���ϴ�����ɣ�Ϊ���Ǽ�������ͭ����������ˮ����ʧ��
��1������ͭ���壨CuSO4?xH2O������ɫ�ģ�ʧȥ�ᾧˮ���Ϊ��ɫ��
��2����������д����ѧ����ʽ��2CuSO4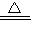 2CuO+2SO2��+O2����
2CuO+2SO2��+O2����
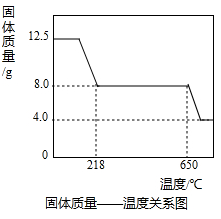
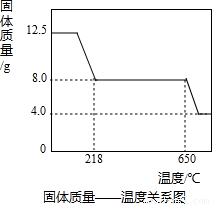
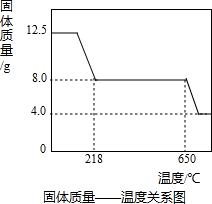
��3����ͼʾ���ݺ͡���t1��ʱ�ù�����ȫʧȥ�ᾧˮ����֪���ᾧˮ������Ϊ��12.50g-8.0g=4.5g
CuSO4?xH2O CuSO4+xH2O����
CuSO4+xH2O����
160+18x 18x
12.50g 4.5g
��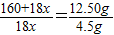
��160+18x=50x��
��x=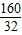 =5��
=5��
CuSO4?5H2O��CuSO4���������� ×100%=64%
×100%=64%
��4.0gʣ�����������ȵ����ߵ��¶ȣ�˵�����ɵ�����ͭ�ֽ��ˣ���ѧ����ʽΪ��4CuO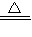 2Cu2O+O2����
2Cu2O+O2����
��Cu4��OH��6SO4��OH���ϼ�Ϊ-1��SO4�Ļ��ϼ�Ϊ-2�����ݻ��������������ϼ۵Ĵ�����Ϊ0���ɵ�ͭԪ�صĻ��ϼ�Ϊ+2��
Cu4��OH��6SO4����д���κͼ����ʽΪCuSO4?3Cu��OH��2��Cu2+���ؽ���ʹ������ʧȥ���ԣ���˵����ԭ���������ǵ����ʣ�
�ʴ�Ϊ��
��1����2Cu+O2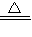 2CuO����CuO+H2SO4=CuSO4+H2O����Cu+H2O2+H2SO4=CuSO4+2H2O��
2CuO����CuO+H2SO4=CuSO4+H2O����Cu+H2O2+H2SO4=CuSO4+2H2O��
��2��2 �����ٻ�����Ⱦ��
��3����ֹH2O2���ȷֽ�
��4��4900��3.06L ��Ũ�������ձ��ڻ�������ˮ�У����ò��������Ͻ���
��5����������ͭ����������ˮ����ʧ
��1���ף�
��2��2CuSO4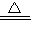 2CuO+2SO2��+O2��
2CuO+2SO2��+O2��
��3��5��64%
��4��4CuO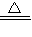 2Cu2O+O2��
2Cu2O+O2��
��+2��CuSO4?3Cu��OH��2 ������
����������Ϊ����ͭ��֪ʶΪ�����������˶���֪ʶ���ѶȽϴ�
 2CuO����CuO+H2SO4�TCuSO4+H2O����Fe+CuSO4�TFeSO4+Cu���������ա��������Ⱦ���������塢�۳������������Բ����ҷ�����������Ȼ�������ȡ���й����⣻���ѧ���ļ���������������ճɹ�����ǿ�ɾУ�
2CuO����CuO+H2SO4�TCuSO4+H2O����Fe+CuSO4�TFeSO4+Cu���������ա��������Ⱦ���������塢�۳������������Բ����ҷ�����������Ȼ�������ȡ���й����⣻���ѧ���ļ���������������ճɹ�����ǿ�ɾУ�����⣺��1��ͭ�ڿ������������ɺ�ɫ��������ͭ������ͭ�����ᷴӦ������ɫ����ͭ������м��Ӧ�û���ͭ����Ӧ����ʽΪ��2Cu+O2
 2CuO��
2CuO��CuO+H2SO4�TCuSO4+H2O�� Fe+CuSO4�TFeSO4+Cu��
��2���ӻ����Ƕȶ��������IJ�ͬ���ֽ��бȽϣ�������һ�����ջ������Ⱦ���������壬�۳����������ɴ˿�֪����2��������
��3��H2O2�����ֽ⣬�¶�Ӧ�ÿ�����50�浽60��֮�䣻
��4����ϡ���������Ϊx����36.8%x=1000mL×1.84g/mL×98%����x=4900g����Ҫˮ���������4900g-1000mL×1.84g/mL��÷1.0g/mL=3060mL����3.06L��
ϡ��Ũ����ķ����ǣ���Ũ�������ձ��ڻ�������ˮ�У����ò��������Ͻ��裮
��5������ͭ����������ˮ��������95%�ľƾ���ϴ�����ɣ�Ϊ���Ǽ�������ͭ����������ˮ����ʧ��
��1������ͭ���壨CuSO4?xH2O������ɫ�ģ�ʧȥ�ᾧˮ���Ϊ��ɫ��
��2����������д����ѧ����ʽ��2CuSO4
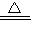 2CuO+2SO2��+O2����
2CuO+2SO2��+O2������3����ͼʾ���ݺ͡���t1��ʱ�ù�����ȫʧȥ�ᾧˮ����֪���ᾧˮ������Ϊ��12.50g-8.0g=4.5g
CuSO4?xH2O
 CuSO4+xH2O����
CuSO4+xH2O����160+18x 18x
12.50g 4.5g
��
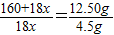
��160+18x=50x��
��x=
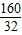 =5��
=5��CuSO4?5H2O��CuSO4����������
 ×100%=64%
×100%=64%��4.0gʣ�����������ȵ����ߵ��¶ȣ�˵�����ɵ�����ͭ�ֽ��ˣ���ѧ����ʽΪ��4CuO
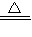 2Cu2O+O2����
2Cu2O+O2������Cu4��OH��6SO4��OH���ϼ�Ϊ-1��SO4�Ļ��ϼ�Ϊ-2�����ݻ��������������ϼ۵Ĵ�����Ϊ0���ɵ�ͭԪ�صĻ��ϼ�Ϊ+2��
Cu4��OH��6SO4����д���κͼ����ʽΪCuSO4?3Cu��OH��2��Cu2+���ؽ���ʹ������ʧȥ���ԣ���˵����ԭ���������ǵ����ʣ�
�ʴ�Ϊ��
��1����2Cu+O2
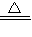 2CuO����CuO+H2SO4=CuSO4+H2O����Cu+H2O2+H2SO4=CuSO4+2H2O��
2CuO����CuO+H2SO4=CuSO4+H2O����Cu+H2O2+H2SO4=CuSO4+2H2O����2��2 �����ٻ�����Ⱦ��
��3����ֹH2O2���ȷֽ�
��4��4900��3.06L ��Ũ�������ձ��ڻ�������ˮ�У����ò��������Ͻ���
��5����������ͭ����������ˮ����ʧ
��1���ף�
��2��2CuSO4
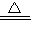 2CuO+2SO2��+O2��
2CuO+2SO2��+O2����3��5��64%
��4��4CuO
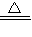 2Cu2O+O2��
2Cu2O+O2����+2��CuSO4?3Cu��OH��2 ������
����������Ϊ����ͭ��֪ʶΪ�����������˶���֪ʶ���ѶȽϴ�

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ
 ij��ҵ��һ���������������ƷΪ���ĵط�������ҵ���ڵ������������У����ɱ���ػ����һ�����ĺ�ͭ���ϣ��磺������£���ij��ѧ��ȤС���ͬѧ��֪��һ�������������ú�ͭ�����Ʊ�������CuSO4?5H2O����
ij��ҵ��һ���������������ƷΪ���ĵط�������ҵ���ڵ������������У����ɱ���ػ����һ�����ĺ�ͭ���ϣ��磺������£���ij��ѧ��ȤС���ͬѧ��֪��һ�������������ú�ͭ�����Ʊ�������CuSO4?5H2O����