��Ŀ����
�����⻯�����⻯�ƣ�CaH2�����⻯�ƣ�NaH����һ����Ҫ�����������ˮ�Ӵ�ʱ���ֱ������·�Ӧ�� CaH2+2H2O=Ca��OH��2+2H2�� NaH+H2O=NaOH+H2��
��1����ij��ѧ��Ӧ��Ҫ����10g��������Ҫ���Ķ��ٿ�NaH��
��2����ɽ�˶�Ա��ɽʱҲ��ͨ�������⻯����ˮ��Ӧ����������ṩ�����������С����Ϊ������CaH2���NaH�������ڵ�ɽ�˶�Ա�������������Ϊʲô��
��1����ij��ѧ��Ӧ��Ҫ����10g��������Ҫ���Ķ��ٿ�NaH��
��2����ɽ�˶�Ա��ɽʱҲ��ͨ�������⻯����ˮ��Ӧ����������ṩ�����������С����Ϊ������CaH2���NaH�������ڵ�ɽ�˶�Ա�������������Ϊʲô��
�⣺��1������10g����������NaH������Ϊx
NaH+H2O=NaOH+H2��
24��������������2
x��������������10g
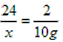
x=120g
��2�����ݻ�ѧ����ʽ��֪��ÿ����4����������������Ҫ���ĵ��⻯�Ƶ�������42�ݣ�������2������������ʱ�����ĵ��⻯�Ƶ�������24�ݣ���Ȼ�����ɵ�����������ʱ�������ĵ��⻯�Ƶ��������⻯�Ƶĸ�С��
NaH+H2O=NaOH+H2��
24��������������2
x��������������10g
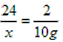
x=120g
��2�����ݻ�ѧ����ʽ��֪��ÿ����4����������������Ҫ���ĵ��⻯�Ƶ�������42�ݣ�������2������������ʱ�����ĵ��⻯�Ƶ�������24�ݣ���Ȼ�����ɵ�����������ʱ�������ĵ��⻯�Ƶ��������⻯�Ƶĸ�С��

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
�����Ŀ