��Ŀ����
ijʵ��̽��С���ͬѧ�������ͼװ�òⶨˮ���⡢��Ԫ�ص������ȣ�����������һ��̽��

��1��д��ͼ��a���������� ʹ�ø�������һ��ע������ ��
��2������Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ ����װ�û���������ʵ������ȡ ���壨д��һ�ּ��ɣ���
��3��ʵ��ʱ��Ӧ��ͨһ��ʱ���H2���ټ�������ͭ��Ŀ���� ��
��4�����Cװ�õIJ���������Ʒ��ʵ��ǰ�������1.6g��Dװ��������1.85g����ʵ���������ˮ��H��OԪ�ص�������Ϊ ����Ϊ�����ȣ���ͬѧ�Ƿ���������ó��Ľ�����Dz��䣬��װ�õ������Լ����еIJ�������ȷ�ģ�����쳣��ԭ���� ��

��1��д��ͼ��a���������� ʹ�ø�������һ��ע������ ��
��2������Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ ����װ�û���������ʵ������ȡ ���壨д��һ�ּ��ɣ���
��3��ʵ��ʱ��Ӧ��ͨһ��ʱ���H2���ټ�������ͭ��Ŀ���� ��
��4�����Cװ�õIJ���������Ʒ��ʵ��ǰ�������1.6g��Dװ��������1.85g����ʵ���������ˮ��H��OԪ�ص�������Ϊ ����Ϊ�����ȣ���ͬѧ�Ƿ���������ó��Ľ�����Dz��䣬��װ�õ������Լ����еIJ�������ȷ�ģ�����쳣��ԭ���� ��
��1���ƾ��ƣ���Ҫ���촵��ƾ��ƵĻ��棨�������ɣ�
��2��Zn + H2SO4 ="==" ZnSO4 + H2����O2 (��CO2) ��3���ž�װ���ڵĿ�������ֹ����ʱ��ը��
��4��25:160����5:32�� �������е�ˮ��������Dװ�ã�ʹˮ������ƫ�Ӷ���Ԫ�ص�����ƫ��
��2��Zn + H2SO4 ="==" ZnSO4 + H2����O2 (��CO2) ��3���ž�װ���ڵĿ�������ֹ����ʱ��ը��
��4��25:160����5:32�� �������е�ˮ��������Dװ�ã�ʹˮ������ƫ�Ӷ���Ԫ�ص�����ƫ��
�����������1��a�Ǿƾ��ƣ�ʹ�õ�ʱ�������촵�𣻣�2����ӦΪп��ϡ���ᷴӦ��������п��������������������ȡ��������Ӧ��Ϊ����������Һ�Ͷ������̣���3����ͨ���������ž�װ���ڵĿ�������ֹ����ʱ��ը����4������ͭ���ٵ���������Ԫ�ص�������D�����ӵ�����Ϊˮ������������ˮ��H��OԪ�ص�������Ϊ5:32�����ܲ�������ԭ���ǿ����е�ˮ��������Dװ�ã�ʹˮ������ƫ�Ӷ���Ԫ�ص�����ƫ��
����������һ���dz����͵�̽���⣬�ص㿼��ķ�Ӧ��˼�룬װ�õȣ�����������Ŀ��Ҫ���£���ϸ���⼴�ɡ�

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ

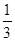 �����NaOH��Һ����������ƿ���С��������֤��CO2��NaOH�����˷�Ӧ��
�����NaOH��Һ����������ƿ���С��������֤��CO2��NaOH�����˷�Ӧ��



