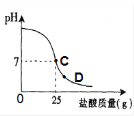
��Ŀ����
����Ŀ����ѧ����������ʶ���ߵ�����
�� ������������ͧ�������Ļ�ѧʽ�� ��1�� ��
�� �����������Ҫ������ ��2�� ��
�� ��ľ����һ�ֳ����� ��3�� �ʣ�����������ס��ء���������Ҫ�ɷֵĻ�ѧʽ�� ��4����
�� �״���CH4O������;֮һ����ȼ�ϣ�CH4O�� ��5�� ��Ԫ����ɣ���Ħ�������� ��6�� ��0.5 molCH4O��Լ������7�� ��H����ѧ�ҷ���һ�������¶�����̼��������Ӧ�����ɼ״�����Ӧ�Ļ�ѧ����ʽΪ��CO2 + ��H2![]() ��CH4O + ��H2O �����ڡ�������������ƽ���ϵ�� ��8��
��CH4O + ��H2O �����ڡ�������������ƽ���ϵ�� ��8��
���𰸡���1��He����2��SO2����3���أ���4��K2CO3�� ��5��3�� ��6��32 g/mol��
��7��1.204��1024�� ��8��1��3��1��1��
��������
����������� �������ɺ�ԭ�ӹ��ɣ������Ļ�ѧʽ��He
�� �����������Ҫ�����Ƕ�������SO2��
�� ������������Ԫ�ط��࣬���е����ס����е�����Ԫ�ؾ������ַ��ϣ�����Ҫ�ɷֵĻ�ѧʽ��K2CO3������Ԫ�أ�Ϊ�طʣ�
�� CH4O�ɣ�C��H��O����Ԫ����ɣ��������Ħ��������ֵ�ϵ�����Է�������������CH4O��Ħ��������32 g/mol��1molCH4O��6.02��1023��CH4O���ӣ���һ��CH4O��������4��Hԭ�ӣ�0.5 molCH4O��Լ���и�0.5��4��6.02��1023������1.204��1024��Hԭ�ӡ���Ӧ�Ļ�ѧ����ʽΪCO2 + 3H2![]() CH4O + H2O
CH4O + H2O

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�