��Ŀ����
��3�֣���������ƣ�Na2S2O3����һ����;�㷺�����ʡ�ij�����������Ʒ�к��������������ơ���ȡ16 g����Ʒ�����ձ��У�����113.6 gһ����������������ϡ����ǡ����ȫ��Ӧ���õ�120 g�����Ʋ�������Һ��
������Ӧ�Ļ�ѧ����ʽΪ��Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2��
����㣺
��1����Ʒ����������ƣ�Na2S2O3���������Ƶ������ȡ�
��2��������Һ����������������
��1��79��1 ��2��12%
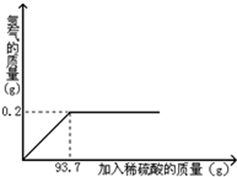
���������������1�����������غ㶨�ɿ����жϣ�����S��SO2��������=16g+113.6g-120g=9.6g���ٸ��ݻ�ѧ����ʽ��Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2����S��SO2��������=1��2������S������Ϊx����SO2������Ϊ2x������X+2x=9.6g��x=3.2g
�⣺ ���������������Ϊy�����ɵ�����������Ϊz
Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2��
158 142 32 64
y z 3.2 g
y="15.8" g z="14.2" g
��1����Ʒ������������������Ƶ�������Ϊ��15.8 g : (16 g - 15.8 g) = 79:1
��2����������Һ��������������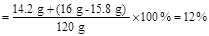
�𣺣�1����Ʒ������������������Ƶ�������Ϊ79:1��
��2����������Һ��������������Ϊ12%��
���㣺���ݻ�ѧ����ʽ���еļ���

��7�֣�ijͬѧ��������ʵ�飺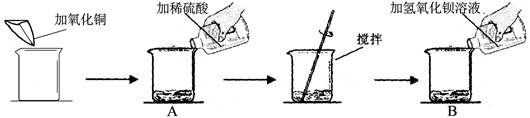
ʵ�����ݼ�����ʵ���������±���
| | ��һ��[��Դ:ѧ���ƣ���Z��X��X��K] | �ڶ��� |
| ������ͭ��������g�� | m | m |
| ��ϡ�����������g�� | 50 | 100 |
| ������������Һ��������g�� | 100 | 100 |
| B����Ҫ���� | ����ɫ���� | �� |
1��д����һ����������Һ��ɫ�ı�Ļ�ѧ��Ӧ����ʽ ��
2���ڶ���ʵ��B�е���Ҫ������ ��
3�������м�������ͭm����ֵΪ ��
4�����ڶ��η�Ӧ�����ɹ���������X���ı���ʽ ��
5�������ڶ��η�Ӧ�����Һ����32��35��ˮ�������ò�������Һ�����ʵ���������Ϊ ;
6������98%��Ũ���������������������ᣬ����Ҫ��ˮ������Ϊ ��
��8�֣�
ij��ѧ��ȤС��ʹ����ͼ��ʾװ�ã���ij��пͭ�Ͻ�ijɷֽ��в�������ȡ����ϡ�������ձ��У��������м���15��0g�Ͻ���Ʒ��ʼ��ʱ������������ƽ�Ķ�����¼���±��У�������м��㣺
��1����Ӧ��ȫ����������������Ƕ��٣�
��2��пͭ�Ͻ���ͭ�������Ƕ��٣�
��3����Ӧ��ȫ����Һ�����ʵ����������Ƕ��٣�
| | ���ձ� | ��������� | ����Ͻ�� 5���� | ����Ͻ�� 10���� | ����Ͻ�� 30���� |
| ������g�� | 21��3 | 169��7 | 184��6 | 184��3 | 184��3 |
��12�֣�ˮ������֮Դ������Ȼ������Ҫ�����ʡ�
����Ȫ�����������ǿ������ȵ�һ����ʽ���Ͼ���ɽ��Ȫ�����ƾõ��Ļ���
��1���峺����Ȫˮ�� ����������������
��2��������Ȫˮ�Ĺ����У���ʹ�õ�����̿����Ҫ�������� ���á�
��3��������Ȫˮ��Ӳˮ������ˮ�������Լ��� ��
��ˮ�Ǿ����Դ���⡣��ˮ��ɹ�ɵõ����Σ���֪ij������Ʒ�г���ɳ�����Na2SO4��MgCl2��CaCl2�����ʡ�ʵ�����ᴿ�������£� 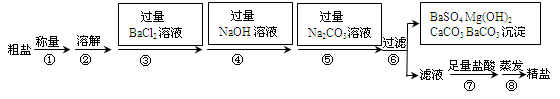
��1����������ƽ����10.2g������Ʒʱ����ָ��ƫ����ߣ����ʾ������ĸ��ţ� ��
| A�������أ������� | B�������ᣬ��Ʒ�� |
| C�������أ���Ʒ�� | D�������ᣬ������ |
��3���ڢݲ�������Ŀ���� ��
��4���ڵڢ߲������У�����Һ�еμ����������Ŀ���� ��
��ˮҲ��һ����Ҫ��ԭ�ϡ�ij�����õ��ˮ�ķ�������ȡ����������ȡ100kg����������������ˮ�������Ƕ��٣�(д���������)