��Ŀ����
��9�֣�ʵ������һƿ������Һ����ʦ��С��ͬѧ��Ʒ����ⶨ�÷�Һ�����������������С��ͬѧ��ȡһ�ྻС�ձ�����������Ϊ18.2g��Ȼ�������е������������Һ�������������Ϊ33.2g��֮��һö����Ϊ10.8g������������ɰֽ��ĥȥ�����⣩�����С�ձ��з�Ӧ�����������治�������ݲ������ٴγ�����������Ϊ43.9 g��
��ش��������⣺
��1����Ӧ�в���������������� ��
��2������÷�Һ�����������������д��������̣�����������һλС��������6�֣�
��3���������������δ�������Լ�������Ӱ���� (ѡ�ƫ����ƫС��������Ӱ�족)��ԭ���� ��
��1��0.1g ��2��32.7% ��3��ƫС�����������ⷴӦ
��������������Ÿ��������غ㶨�ɣ���������������Ϊ��33.2g+10.8g-43.9g=" 0.1g"
(2)(6��) �⣺���Һ��H2SO4����Ϊx
Fe + H2SO4 �� FeSO4 + H2��
98 2
X 0.1g
98/X=2/0.1g
x="4.9" g
��Һ��������������� ��4.9g/(33.2g-18.2g)*100% ��32.7��
���������������δ�������Լ�������Ӱ����ƫС,��Ϊ���������ⷴӦ������ɸ�����������������������ƫ�٣��̶���ɷ�Һ���������������ļ���ƫС��
���㣺�����غ㶨�ɡ��йػ�ѧ����ʽ�ļ��㡢�������������ļ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���7�֣��кͷ�Ӧ����ѧ��ѧ����Ҫ��ѧϰ���ݣ������ճ������ũҵ�������й㷺��Ӧ�á�
��1����ͼ��ʾ���������������Һ������Ӧ��������Һ��pH�ı仯���ߡ��������ͼ�л�ȡ��Ϣ���ش��������⣺
��ͼ1ͼ���ʾ���������������Һ������Ӧ��������Һ��pH�仯�����и÷�Ӧ��ʵ������ǰ���ͼ2�е� ������ң�ͼ��ʾ���еġ�
��������M���ʾ ��
�����ձ����㵹20g��������Ϊ4.00��������������Һ������3�η�̪��Һ��������ε�����������Ϊ3.65����ϡ���ᣬ�ߵα���ֱ����Һ�պñ�Ϊ ɫΪֹ������ȥϡ����20g����Ӧ����Һ��������������Ϊ (�����ȷ��0.1��)��
��2��Ϊ֤���кͷ�Ӧ�Ƿ��ȷ�Ӧ��ijС���������ͼ��
ʾ��ʵ�������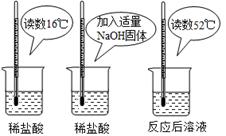
������ͼʵ�飬��ͬѧ��Ϊ��NaOH��ϡ���ᷢ�����кͷ�Ӧ�Ƿ��ȷ�Ӧ����ͬѧ��Ϊ����ͬѧ�ó�
������۵����ݲ���ѧ�������� ��
��3��Ϊ̽��Ӱ���кͷ�Ӧ�ų��������ٵ����أ������ֽ���������ʵ�飺�ڱ��ΪA��B��C��
D��E����ֻ�ձ��и�װ��36.5g ������������Ϊ5%��10%��15%��20%��25%�����ᣬ
����������ֻ�ձ��зֱ����40g20% ������������Һ�����������¶ȣ����ݼ�¼���£�
| �ձ���� | A | B | C | D | E |
| ����������������� | 5% | 10% | 15% | 20% | 25% |
| ��Ӧ����Һ�¶ȣ��棩 | 24�� | 34�� | 46�� | 54�� | 54�� |
������˼����Ӧ���ձ�����ҺpH��С���� �����ձ���ţ���
