��Ŀ����
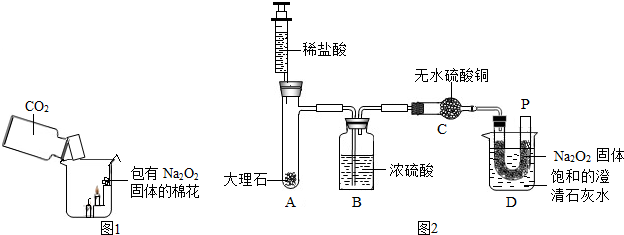
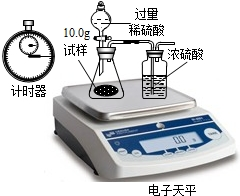
ij��ѧ��ȤС����15gʯ��ʯ��Ʒ�����ʲ�����ˮ�Ҳ����ᷴӦ��������������ϡ���ᷴӦ������ʵ��̽����ʵ��װ�����£�

����ʾ����ʯ�ҵijɷ�Ϊ�����ƺ��������ƣ������ն�����̼��ˮ�����Լ�HCl���壩��Aװ�������������ʱ�����Bװ�õ�����������4.7g��Cװ�õ�����û�����仯��������������ش�
��1��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊ
��2����Aװ�������������ʱ�����ĵ�ϡ����
��3��15g��Ʒ����ܲ���������̼������������ٿˣ���д��������̣�
��4��������ϡ���������������������д��������̣�

����ʾ����ʯ�ҵijɷ�Ϊ�����ƺ��������ƣ������ն�����̼��ˮ�����Լ�HCl���壩��Aװ�������������ʱ�����Bװ�õ�����������4.7g��Cװ�õ�����û�����仯��������������ش�
��1��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊ
10
10
g����2����Aװ�������������ʱ�����ĵ�ϡ����
100
100
g����3��15g��Ʒ����ܲ���������̼������������ٿˣ���д��������̣�
��4��������ϡ���������������������д��������̣�
��������1��ͨ���۲�A��ʣ����������ͼ�������Ĺ�ϵ��֪���������������������ʱ��ʣ�������������ڼ��٣��������֪ʣ����������ʣ����ʵ�������5g�����֪ʯ��ʯ��Ʒ��̼��Ƶ�������
��2����̼��Ƶ�����������̼��ƺ����ᷴӦ�ķ���ʽ����ʾ��������ϵ���Ϳ������15g��Ʒ��������ɶ�����̼���������������������μӷ�Ӧ��ϡ���������ʵ��������������غ㶨�ɿ�֪���ĵ�ϡ���������=��Ӧ���Aװ�������ʵ�������+������̼������-ʯ��ʯ��Ʒ���������н��
��3����̼��Ƶ�����������̼��ƺ����ᷴӦ�ķ���ʽ����ʾ��������ϵ���Ϳ������15g��Ʒ��������ɶ�����̼������������н��
��3�����������غ㶨�ɣ��ɷ�Ӧ����Һ���������μӷ�Ӧ��̼��Ƶ����������ɵĶ�����̼����������������μӷ�Ӧ��������Һ���������ڸ������ʵ����������ļ��㹫ʽ�Ϳ��������ϡ�������������������
��2����̼��Ƶ�����������̼��ƺ����ᷴӦ�ķ���ʽ����ʾ��������ϵ���Ϳ������15g��Ʒ��������ɶ�����̼���������������������μӷ�Ӧ��ϡ���������ʵ��������������غ㶨�ɿ�֪���ĵ�ϡ���������=��Ӧ���Aװ�������ʵ�������+������̼������-ʯ��ʯ��Ʒ���������н��
��3����̼��Ƶ�����������̼��ƺ����ᷴӦ�ķ���ʽ����ʾ��������ϵ���Ϳ������15g��Ʒ��������ɶ�����̼������������н��
��3�����������غ㶨�ɣ��ɷ�Ӧ����Һ���������μӷ�Ӧ��̼��Ƶ����������ɵĶ�����̼����������������μӷ�Ӧ��������Һ���������ڸ������ʵ����������ļ��㹫ʽ�Ϳ��������ϡ�������������������
����⣺��1����A��ʣ����������ͼ�������Ĺ�ϵ��֪�����ʵ�������5g��ʯ��ʯ��Ʒ��̼��Ƶ�������15g-5g=10g�����10��
��2����15g��Ʒ�е�̼�����ȫ�����ᷴӦʱ���ɵĶ�����̼��࣬
�����ɶ�����̼������Ϊx���μӷ�Ӧ�����������ʵ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
10g y x
=
x=4.4g
=
y=7.3g
�������⣬A�����µ�����110.3��Ӧ�����������������ʯ��ʯ��Ʒ��������ȥA�лӷ��ߵ�ˮ�Ͷ�����̼����������B���ص�����4.7�ˣ�������ʽΪ��m2+15g-4.7g=110.3g��m2=100g��
��4������ϡ�������������������
��100%=7.3%��
�𣺣�2����Aװ�������������ʱ�����ĵ�ϡ����100g����3��15g��Ʒ��������ɶ�����̼���������Ϊ 4.4g����4������ϡ�������������������7.3%��
��2����15g��Ʒ�е�̼�����ȫ�����ᷴӦʱ���ɵĶ�����̼��࣬
�����ɶ�����̼������Ϊx���μӷ�Ӧ�����������ʵ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
10g y x
| 100 |
| 10g |
| 44 |
| x |
x=4.4g
| 100 |
| 10g |
| 73 |
| y |
y=7.3g
�������⣬A�����µ�����110.3��Ӧ�����������������ʯ��ʯ��Ʒ��������ȥA�лӷ��ߵ�ˮ�Ͷ�����̼����������B���ص�����4.7�ˣ�������ʽΪ��m2+15g-4.7g=110.3g��m2=100g��
��4������ϡ�������������������
| 7.3g |
| 100g |
�𣺣�2����Aװ�������������ʱ�����ĵ�ϡ����100g����3��15g��Ʒ��������ɶ�����̼���������Ϊ 4.4g����4������ϡ�������������������7.3%��
������������Ҫ�������й��������������뻯ѧ����ʽ���ۺϼ��㣬������ѵ��Ƿ�Ӧǰ���й���Һ�����ķ����ж����ж������ݾ��������غ㶨�ɣ�

��ϰ��ϵ�д�
�����Ŀ