��Ŀ����
����Ŀ�������г������ʵ������Ʒ����ǻ�ѧ�о�����Ҫ���ݡ�

��1�����Ϸ�Ӧ������������϶�������______����������������������������
��2��������ˮ��Ӧ�����������ƺ���������ѧ����ʽΪ______��
����ͼ1ʵ�������в������ڼ��ȵ���______������ĸ���ţ���
a���ձ� b����Ͳ c���Թ� d��������
��3��ij��ѧС��ѡ��ͼ1װ�ú�ҩƷ����̽��ʵ�顣
��A�з�Ӧ�Ļ�ѧ����ʽΪ______��
��������ͼ1װ����ȡ���ռ�һƿ����������Ķ�����̼���壬����ѡ����ʵ�װ�ò�д�����ܿ����ӵ�˳��______��
��4����ͼ2װ�ÿ���һ����̼��ԭ��������ʵ�飬���������ɵ�������
��Bװ�ò�������ɹ۲쵽��������______��
��Cװ���з�Ӧ�Ļ�ѧ����ʽΪ______��
��5����30.6g����أ�KClO3���Ͷ������̵Ĺ�������װ���Թ��У�������ȡ������ͬʱ�����Ȼ��ء�����Ӧ��ȫ���Թ���ȴ�����������Եõ�21.0g�������ʡ������ԭ��������������ص���������______��
���𰸡����� 2Na+2H2O=2NaOH+H2�� b CaCO3+2HCl=CaCl2+H2O+CO2�� agfedh ��ɫ������ɫ Ca��OH��2+CO2=CaCO3��+H2O 80.1%
��������
��1�����Ϸ�Ӧ������������϶������ǵ��ʡ�
������ʡ�
��2��������ˮ��Ӧ�����������ƺ���������ѧ����ʽΪ��2Na+2H2O=2NaOH+H2����
���2Na+2H2O=2NaOH+H2����
����ͼ1ʵ�������в������ڼ��ȵ�����Ͳ��
���b��
��3����A��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
���CaCO3+2HCl�TCaCl2+H2O+CO2����
��������ͼ1װ����ȡ���ռ�һƿ����������Ķ�����̼���壬���ܿ����ӵ�˳��a��g��f����ȥ�Ȼ��⣩��e��d����ȥˮ��������h��
���agfedh��
��4����Bװ�ò���������������һ����̼��Ӧ�������Ͷ�����̼���ɹ۲쵽��ɫ������ɫ��
�����ɫ������ɫ��
��Cװ�����������ƺͶ�����̼��Ӧ����̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2�TCaCO3��+H2O��
���Ca��OH��2+CO2�TCaCO3��+H2O��
��5�������������Ϊx��
��Ӧ��������������30.6g-21.0g=9.6g��
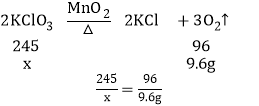
x=24.5g��
ԭ��������������ص�����������![]() ��100%=80.1%��
��100%=80.1%��
��ԭ��������������ص���������80.1%��

 �Ķ��쳵ϵ�д�
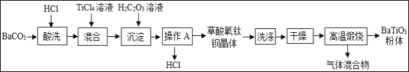
�Ķ��쳵ϵ�д�����Ŀ����ѧѧ��ʵ������ϣ���ʦ����һ����ĩ�������ɳ��ڷ����ڿ����е����ۺ;��õļ�ʯ�Ҹ������϶��ɣ���ͬѧ�����ʵ�����̽����
��������⣩������Ʒ�ijɷ���ʲô��
���������ϣ���1����ʯ�Ҹ������CaO��NaOH�Ļ���
��2��BaCl2��Һ�����ԡ�
����������裩������Ʒ�п��ܺ��� Fe��Fe2O3��CaO��NaOH��Ca��OH��2��Na2CO3��_____���������ʡ�
������ʵ�飩

����ͬѧ������B�ijɷֽ���̽������¼���£�
ʵ����� | ʵ������ | ʵ����ۼ���ѧ����ʽ |
ȡ��������B���Թ��У���������ϡ���ᣬ�����ɵ�����ͨ������ʯ��ˮ�� | ��_____ ��_____ | ���ۣ�����B���� Fe2O3��CaCO3��ʵ������з�Ӧ�Ļ�ѧ����ʽΪ��_____�����һ�����ɣ� |
����ͬѧ����ҺC�ijɷֽ���̽������¼���£�
ʵ����� | ʵ������ | ʵ����� |
ȡ������ҺC���Թ��У��������BaCl2��Һ�����ã����ϲ���Һ�еμ���ɫ��̪��Һ���� | ���а�ɫ�������� �ڷ�̪��Һ����ɫ��ɺ�ɫ | ��ҺC����_____ |
����������ۣ���1������ͬѧʵ���м������BaCl2��Һ��Ŀ����_____��
��2��ͨ������ʵ��̽�����ù�����Ʒ����ȷ��һ�����еijɷ���_____��