��Ŀ����
����Ŀ����ѧ���������ϢϢ��أ������û�ѧ֪ʶ�ش��������⣮
��1������ʹ��Ӳˮ���������������������鷳,���dz�����_________������Ӳˮ����ˮ��
��2���������еļ��ȼ��Ƿֿ���װ����ʯ�Һ�ˮ��ʹ��ʱ�������Ͻ������ͻᷢ����Ӧ���ų��������÷�Ӧ�Ļ�ѧ����ʽΪ______________��
��3������ʯ�ҷ�ˢǽ�ڣ���ÿ����÷�Ӧ�Ļ�ѧ����ʽΪ_______________________
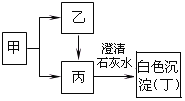
��4��ú���������������������Ҫ����Դ����ҵ�ϳ���ú����������Һ��������ʹú��������Դ��ú������Һ������ʾ��ͼ����ͼ��ʾ��

�ڢٲ�������������____�仯(���������ѧ��)��
�ڢڲ��Ǿ���ú��ˮ�����ķ�Ӧ����ѧ����ʽΪ______________________��
��2.8tCO������H2��ַ�Ӧ�������Ͽ��Եõ��״�______ t��
��5���廪��ѧ��ѧ����ѧԺ�о���Ա�ɹ����Ƴ�һ��������ά�������ɽ�������̼ת����Һ��ȼ�ϼ״�������ʾ��ͼ���£����ڷ����ڲ�ȫ��Ӧ����ͼʾ��
![]() ______
______
���𰸡�����ˮ CaO+H2O==Ca(OH)2 Ca(OH)2+CO2==CaCO3��+H2O ���� C + H2O![]() CO + H2 3.2t
CO + H2 3.2t ![]()
��������
��1�����������dz����÷���ˮ��������ˮ��Ӳˮ�����ָ����϶����Ӳˮ��û�и����������ٵ�����ˮ���������ˮ��
��2��������ʯ�Һ�ˮ��Ӧ�����������Ʒų��������ȣ�����ʽ����CaO+H2O==Ca(OH)2��
��3����ʯ�ҺͿ����еĶ�����̼��Ӧ�������ܵ�̼��ƺ�ˮ������ʽ����Ca(OH)2+CO2=CaCO3��+H2O��
��4��
����ú��ϴѡ�ӹ��������仯������������
�ھ���ú��ˮ������Ӧ����һ����̼������������ʽ����C + H2O![]() CO + H2��һ����̼�������ڴ��������������ɼ״�������ʽΪCO+2H2
CO + H2��һ����̼�������ڴ��������������ɼ״�������ʽΪCO+2H2![]() CH3OH�����ݻ�ѧ����ʽ��������ɵõ��״�����Ϊx��
CH3OH�����ݻ�ѧ����ʽ��������ɵõ��״�����Ϊx��

![]() =
= ![]()
���x=3.2t������3.2t��
��5������ͼ��֪�μӷ�Ӧ�ķ�Ӧ����3���������6����ԭ������1��������̼������1��̼ԭ�Ӻ�2����ԭ�ӣ�����������1���״����ӣ�1��̼ԭ�ӡ�1����ԭ�Ӻ�4����ԭ���������������غ㶨�ɿ�֪�ڻ�ѧ��Ӧǰ��ԭ�ӵ���������Ŀ�������������������л���1����ԭ�Ӻ�2����ԭ�ӣ�����![]() ��
��

 �������Ͽ��㱾ϵ�д�
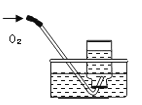
�������Ͽ��㱾ϵ�д�����Ŀ��������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ���õ���( )
ѡ�� | A | B | C | D |
ʵ��װ�� |
����������ȼ�� |
�ⶨ�������������� |
��˿��������ȼ�� |
��ˮ���ռ����� |
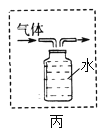
���� | ����ƿ�е�ˮ���շų������� | ��Ͳ�е�ˮ��ͨ��ˮ����ı仯�ó�O2��� | ����ƿ�е�ˮ����ȴ�����������ֹ����ƿը�� | ����ƿ�е�ˮ����ˮ������ƿ�ڵĿ����ž����ڹ۲�O2��ʱ�ռ��� |
A. A B. B C. C D. D