��Ŀ����
����Ŀ������۱�ʶ����̽�����ǻ�ѧ��������֮һ��
��1����Ϥ��ѧ������ѧϰ��ѧ�ıر����ߣ���ȷд�����л�ѧ���
������_____��
������ͭ��ͭԪ�صĻ��ϼ�Ϊ+2��_____��
������ˮ�Ļ�ѧ���ʵ���С��_____��
��������_____��
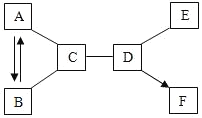
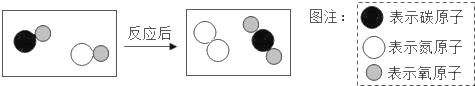
��2��������̼ת��Ϊ�״���CH3OH���ķ�Ӧ��ʾ��ͼ��ͼ��
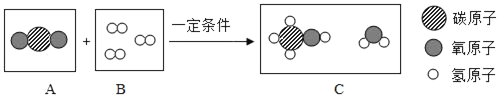
��B���е���������_____��ѡ��������������������������ʡ�����
���μӷ�Ӧ��A��B���Ӹ�����Ϊ_____��
���÷�Ӧ�Ļ�ѧ����ʽ_____��
���𰸡�Ne ![]() H2O Fe2��SO4��3 ���� 1��3 CO2+3H2
H2O Fe2��SO4��3 ���� 1��3 CO2+3H2![]() CH3OH+H2O
CH3OH+H2O
��������
��1������������ԭ��ֱ�ӹ��ɵģ���ѧʽ�ǣ�Ne��
������ͭ��ͭԪ�صĻ��ϼ�Ϊ+2�ۣ�д������ͭ�Ļ�ѧʽ����ͭԪ�ص����Ϸ����+2������Ϊ��![]() ��
��
������ˮ�Ļ�ѧ���ʵ���С����ˮ���ӣ���ѧ�ǣ�H2O��
���������Ļ�ѧʽ�ǣ�Fe2��SO4��3��
��2���ɷ�Ӧ��ʾ��ͼ��֪��Ӧ���������Ͷ�����̼���������Ǽ״���ˮ����˷�Ӧ�ķ���ʽΪ��CO2+3H2![]() CH3OH+H2O��
CH3OH+H2O��
��B���е���������һ�ַ��ӹ��ɵģ���������ͬ�ֵ�ԭ�ӹ��ɵģ����ڵ��ʡ�
���ɷ���ʽ��֪���μӷ�Ӧ��A��B���Ӹ�����Ϊ1��3��
��������������֪���÷�Ӧ�ķ���ʽΪ��CO2+3H2![]() CH3OH+H2O��
CH3OH+H2O��
�ʴ�Ϊ����1����Ne�� ��![]() �� ��H2O�� ��Fe2��SO4��3����2�������ʣ� ��1��3�� ��CO2+3H2
�� ��H2O�� ��Fe2��SO4��3����2�������ʣ� ��1��3�� ��CO2+3H2![]() CH3OH+H2O��
CH3OH+H2O��

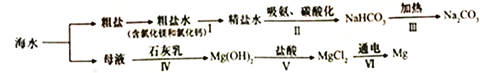
����Ŀ��NH4Cl��Na2SO4���ܽ�ȱ����ܽ���������¡�����˵����ȷ����
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ�� S/g | NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 60.2 |
Na2SO4 | 9.6 | 20.2 | 40.8 | 48.4 | 47.5 | 47.0 | |
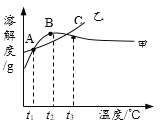
A. ��ΪNH4Cl
B. t3��Ӧ����30����40��
C. �����ʵı�����Һ��t2���µ�t3����Һ������������������
D. �ס��ұ�����Һ��t3���µ�t1���������壨�������ᾧˮ��������һ�����