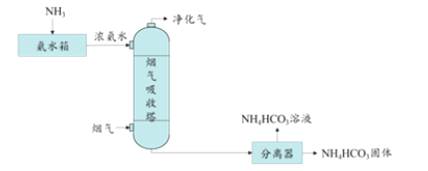
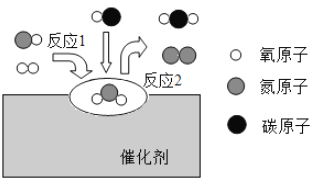
��Ŀ����
����Ŀ��������ҹ���������ʳ����ʷ�ƾá�������õ�һ��ȼ���ǹ���ƾ���ij��ѧ��ȤС���ͬѧ�ԡ�����ƾ��������˺��棬������ɷֽ���������̽������ش��������⣺
���������ϣ�
a������ƾ����þƾ����Ȼ��ƺ��������ư�һ���������Ȼ���Ƴɵġ�
b���Ȼ�����Һ���Ȼ�����Һ�������ԡ�
��������⣩
���ƾ����Ƿ���̼Ԫ�أ�
������ƾ��е����������Ƿ���ʣ�
��ʵ��̽����
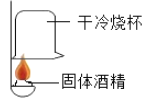
��1��̽���Ҵ�ȼ�յ������
ʵ�鷽�� |
|
|
ʵ������ | �ձ��ڱڳ���ˮ�� | �ձ��ڱ�_____ |
ʵ����� | ˵���ƾ�ȼ������_____ | ˵���ƾ�ȼ�����ɶ�����̼ |
������ʵ��ɵó��ƾ���һ������_____Ԫ�ء�
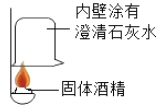
��2��ȡ��������ƾ����ձ��У���������ˮ����ܽ���ã������ձ��ײ��а�ɫ���������û�ѧ����ʽ��ʾ�ó������γɣ�_____���ɴ�˵�����������ѱ��ʡ�
��3��Ϊ��һ��ȷ���������Ƶı��ʳ̶ȣ�ͬѧ�Ƿ������̽����
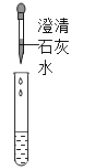
������ͬѧȡ�������ձ��ϲ���Һ����֧�Թ��У�����ͼ��ʾ����ʵ�顣
ʵ�鷽�� |
|
|
ʵ������ | ��Һ��� | ������ɫ���� |
ʵ����� | ��Һ������������ | ��Һ����_____ |
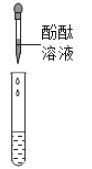
������ͬѧ��Ϊ����ͬѧ��ʵ�鲻��֤����Һ��һ�����������ƣ�������_____������������ȡ�ձ��е��ϲ���Һ�����������Ȼ�����Һ����ַ�Ӧ���ã���ȡ��Ӧ����ϲ���Һ���μӷ�̪��Һ����̪��Һ��졣
����˼������
��4������ͬѧ��ʵ���м��������Ȼ�����Һ��Ŀ����_____��
��ʵ����ۣ�С��ͬѧ�������ۣ�һ����Ϊ�ù���ƾ��е��������Ʋ��ֱ��ʡ�
����չ������С��ͬѧ�������۷��֣�ͨ�����з���Ҳ�����жϹ���ƾ��е����������Ƿ���ʡ�
��5��ȡ����Ʒ�������Թ��У�����������ˮ������Ʒȫ������ˮ���������м���������_____���۲쵽_____��˵����Ʒ��ı����ˡ���ʵ������ط�Ӧ�Ļ�ѧ����ʽΪ_____��
���𰸡�ˮ ����ʯ��ˮ����� ̼���� CaCl2+Na2CO3��2NaCl+CaCO3�� Na2CO3 ̼������ҺҲ�ʼ��ԣ���ʹ��ɫ��̪��Һ��� ��ȥ��Һ��̼���ƣ���ֹ���������Ƶļ�����ɸ��� ���� ������ð�� Na2CO3+2HCl��2NaCl+H2O+CO2��
��������
[ʵ��̽��]
��1�����������غ㶨�ɽ��з������
��2�����ݷ�Ӧ����������д��ѧ����ʽ��
��3���ٸ���ʵ������������
�ڸ����������ƺ�̼������Һ���ʼ��Խ��з������
��4�����ݳ�ȥ̼������ӵĸ��Ž��з������
��5������̼���Ƶ����� ���з������
��1�����������غ㶨�ɿ�֪�����ձ��ڱڳ���ˮ��֪�ƾ�ȼ��������ˮ��˵���ƾ���һ��������Ԫ�أ�֤�����ɶ�����̼�������dz���ʯ��ˮ����ǣ��ƾ��к���̼Ԫ�أ��ʴ�Ϊ��ˮ ����ʯ��ˮ����� ̼���⣻
��2��ȡ��������ƾ����ձ��У���������ˮ����ܽ���ã������ձ��ײ��а�ɫ������������̼��ƣ���Ӧ�����Ȼ��ƺ�̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ��
CaCl2+Na2CO3��2NaCl+CaCO3�����ʴ�Ϊ��CaCl2+Na2CO3��2NaCl+CaCO3����
��3��������Һ�еμӳ���ʯ��ˮ������ɫ������˵����Һ�к���̼���ƣ��ʴ�Ϊ��Na2CO3��
�ڼ���ͬѧ��ʵ�鲻��֤����Һ��һ�����������ƣ���Ϊ̼������ҺҲ�ʼ��ԣ���ʹ��ɫ��̪��Һ��죬�ʴ�Ϊ��̼������ҺҲ�ʼ��ԣ���ʹ��ɫ��̪��Һ��죻
��4�������������Ȼ�����Һ��Ϊ�˳�ȥ��Һ��̼���ƣ���ֹ���������Ƶļ�����ɸ��ţ��ʴ�Ϊ����ȥ��Һ��̼���ƣ���ֹ���������Ƶļ�����ɸ��ţ�
��5������̼�����ܺ����ᷴӦ�������壬����������м��飺ȡ����Ʒ�������Թ��У�����������ˮ������Ʒȫ������ˮ���������м������������ᣬ�۲쵽������ð����˵����Ʒ��ı����ˡ���ʵ������ط�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl��2NaCl+H2O+CO2���ʴ�Ϊ������ ������ð�� Na2CO3+2HCl��2NaCl+H2O+CO2����

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д�