��Ŀ����
С��Ҫ����100g��������Ϊ5%���Ȼ�����Һ����ͼ���������Ȼ�����Һ��ʵ�����ʾ��ͼ��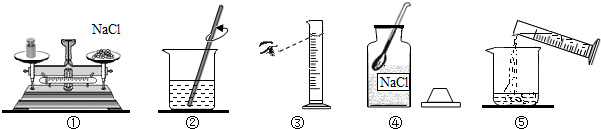
��ش��������⣺
��1����������100g������������Ϊ5%���Ȼ�����Һ���裺�Ȼ���
��2��������ͼʾ����ű�ʾ������Һ�IJ���˳��
��3��ָ��ͼ�е�һ����������
����ʵ���в�ȡ�˲�������õ���ҺŨ�Ȼ�
��4����������ƽ��ȷ����������Ȼ���ʱ������������ƽ��ָ��ƫ�����̣�Ӧ
A�����������Ȼ��ƹ��� B�����������Ȼ��ƹ��� C������ƽ����ĸ��
��������1����������=��Һ���������������������ܼ�����=��Һ����-����������
��2������������Һ�IJ��迼�DZ��⣻
��3��������ƽ����Ͳ��ʹ�ù����ǣ�
��4��������ƽ����ҩƷ��ʹ�ù�����
��2������������Һ�IJ��迼�DZ��⣻
��3��������ƽ����Ͳ��ʹ�ù����ǣ�
��4��������ƽ����ҩƷ��ʹ�ù�����
����⣺��1���Ȼ���������100g��5%=5g����ˮ��100g-5g=95g��
�T95mL��Ӧѡ��100mL��Ͳ��
�ʴ�Ϊ��5��95��100 mL
��2������������������һ������Һ�Ļ������裺���㡢��������ȡ���ܽ⡢װƿ��ţ�Ҫע���ڳ���ǰ��ȡ��ҩƷ���ܳ�����
�ʴ�Ϊ���ܢ٢ۢݢ�
��3������ƽ��������ʱҪ�������룬��ͼʾ��ȴ�����������Ͳ����ʱӦƽ�Ӱ�Һ����ʹ�����ͼ��Ϊ���ӣ����ȡ���Ȼ��������٣�����Ͳ��ȡ��Һ����������õ���ҺŨ�Ȼ�ƫ�ͣ�
�ʴ�Ϊ���������ж���ʱ���ӿ̶ȡ�װ�â����Ȼ����������λ�õߵ��ˣ�ƫ��
��4������������Ȼ��ƣ�������ƽ��ָ��ƫ�����̣�˵��ҩƷ������������Ӧ���������Ȼ��ƹ��壻
��ѡB
| 95g |
| 1g/ml |
�ʴ�Ϊ��5��95��100 mL
��2������������������һ������Һ�Ļ������裺���㡢��������ȡ���ܽ⡢װƿ��ţ�Ҫע���ڳ���ǰ��ȡ��ҩƷ���ܳ�����
�ʴ�Ϊ���ܢ٢ۢݢ�
��3������ƽ��������ʱҪ�������룬��ͼʾ��ȴ�����������Ͳ����ʱӦƽ�Ӱ�Һ����ʹ�����ͼ��Ϊ���ӣ����ȡ���Ȼ��������٣�����Ͳ��ȡ��Һ����������õ���ҺŨ�Ȼ�ƫ�ͣ�
�ʴ�Ϊ���������ж���ʱ���ӿ̶ȡ�װ�â����Ȼ����������λ�õߵ��ˣ�ƫ��
��4������������Ȼ��ƣ�������ƽ��ָ��ƫ�����̣�˵��ҩƷ������������Ӧ���������Ȼ��ƹ��壻
��ѡB
������ͨ���ش���֪�������ʡ��ܼ������ļ��㷽��������ƽ����ʱ��ע���������������������������һ������Һ�Ļ������裮

��ϰ��ϵ�д�
 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д� ����������ϵ�д�
����������ϵ�д�
�����Ŀ
 ��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣���
��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣��� ��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣���
��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣���