��Ŀ����
 ��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣���
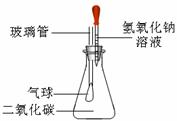
��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣�����1��ijͬѧΪ̽������������Һ�����ʣ���������ͼʵ�飬��10g��������Ϊ40%����������Һ������ƿ���Ը���Ҫ�ش��������⣺
��д���۲쵽��һ��ʵ������
�������
�������
���ڵ�������������Һ����Ӧ����������װ�õ�������֮ǰ��Ƚ�
a
a
����д���и������ţ���a��û�б仯 b���б仯������������С
c���б仯������������� d������ȷ��
��������������Һ��ȫ��Ӧ��������̼����ʣ�࣬��ͨ�����㣬���ʱ���ò�������Һ�����ʵ�������
��2��ijУѧϰС���ͬѧҪ�����Ȼ�����Һ��ҩƷ���пɹ�ѡ���ҩƷ���������ơ������ơ�̼������ֹ��弰һ����������������ϡ���ᣮͬѧ�ǽ������ۺ�ѡ����һ�ֹ���ҩƷ����ȡ�ù���15.4g�����ձ��У��������м���100gϡ���ᣬǡ����ȫ��Ӧ����Ӧ����Һ����Ϊ111g���������Ŀ�����ݵı仯�����ش��������⣺
��ͬѧ��ѡ��Ĺ���ҩƷ��
̼���
̼���
���ڸ�ʵ����̿�����һ������������
��������
��������
������д�����з�����Ӧ�Ļ�ѧ����ʽ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
�����������Ȼ�����Һ��������������Ϊ���٣���д��������̣�
��������1�������ݶ�����̼�����������Ʒ�Ӧ���������ڵ�����ѹǿ��С������𣻢����������غ㶨�ɵ�Ӧ�ý��ͼ��ɣ������ݷ�Ӧ���������Ƶ������������Ӧ���ɵ�̼���Ƶ�������
��2����Ϊ��Ӧǰ�����ʵ���������С�ˣ����Կ���֪����Ӧ���������壬��˿������������Ϊͻ�ƿڣ�������ɣ�
��2����Ϊ��Ӧǰ�����ʵ���������С�ˣ����Կ���֪����Ӧ���������壬��˿������������Ϊͻ�ƿڣ�������ɣ�
����⣺��1�������ݶ�����̼�����������Ʒ�Ӧ������̼���ƺ�ˮ�����Իᵼ�������ڵ�����ѹǿ��С���Ӷ������������
�����������غ㶨�ɿ�֪��Ӧ����Ϊû�����ʶ�ʧ�����Է�Ӧǰ������װ�õ������������䣨�����������µ������ı䣩��
�����ʱ���ò�������Һ�����ʵ�������x��
CO2+2NaOH=Na2CO3+H2O
80 106
10g��40% x
=
x=5.3g
��2������Ϊ��Ӧǰ�����ʵ���������С�ˣ����Կ���֪����Ӧ���������壬�����������ơ������ơ�̼������ֹ��弰ϡ���ᷴӦ��ֻ��̼��ƺ����ᷢ����Ӧ�������壻
�����ڸ÷�Ӧ�в����˶�����̼���壬���Ը�ʵ��������������ݲ�����
��̼��������ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
�ܷ�Ӧ�����ɵĶ�����̼������15.4g+100g-111g=4.4g��
�����ɵ��Ȼ���������x
CaCO3+2HCl=CaCl2+H2O+CO2��
111 44
x 4.4g
=
x=11.1g
���
��100%=10%
�ʴ�Ϊ����1����������𣻢�a���۴�ʱ���ò�������Һ�����ʵ�����5.3g��
��2����̼��ƣ��ڲ������ݣ���CaCO3+2HCl=CaCl2+H2O+CO2�������������Ȼ�����Һ��������������Ϊ10%��
�����������غ㶨�ɿ�֪��Ӧ����Ϊû�����ʶ�ʧ�����Է�Ӧǰ������װ�õ������������䣨�����������µ������ı䣩��
�����ʱ���ò�������Һ�����ʵ�������x��
CO2+2NaOH=Na2CO3+H2O
80 106
10g��40% x
| 80 |
| 10g��40% |
| 106 |
| x |
x=5.3g
��2������Ϊ��Ӧǰ�����ʵ���������С�ˣ����Կ���֪����Ӧ���������壬�����������ơ������ơ�̼������ֹ��弰ϡ���ᷴӦ��ֻ��̼��ƺ����ᷢ����Ӧ�������壻
�����ڸ÷�Ӧ�в����˶�����̼���壬���Ը�ʵ��������������ݲ�����
��̼��������ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
�ܷ�Ӧ�����ɵĶ�����̼������15.4g+100g-111g=4.4g��
�����ɵ��Ȼ���������x
CaCO3+2HCl=CaCl2+H2O+CO2��
111 44
x 4.4g
| 111 |
| x |
| 44 |
| 4.4g |
x=11.1g
���
| 11.1g |
| 111g |
�ʴ�Ϊ����1����������𣻢�a���۴�ʱ���ò�������Һ�����ʵ�����5.3g��
��2����̼��ƣ��ڲ������ݣ���CaCO3+2HCl=CaCl2+H2O+CO2�������������Ȼ�����Һ��������������Ϊ10%��
�����������Ƕ����ʵ������Լ���ѧ����ʽ����Ŀ����⣬���������Ļ�ѧ�仯����ϻ�ѧ����ʽ�Ļ��������ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
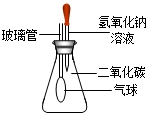 ��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣���
��ѧ��һ���ۺ�ѧ�ƣ�ѧϰ�����м����������ǿ�Ķ��Է������������������������Ķ����������������������������ۺ�Ӧ���⣬��������ѡ������һ�����𣨶�ѡ���ӷ֣���