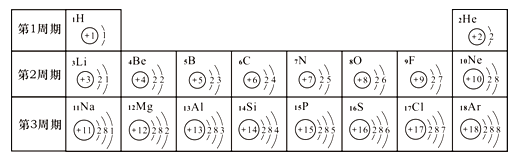
��Ŀ����
����Ŀ��������ÿ������ʴ�����ϵĽ����豸�Ͳ��ϸߴ������20%��40%��ijʵ��С��Խ�����ʴ��������ʵ��̽����
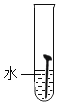
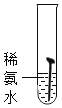
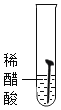
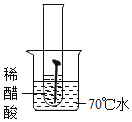
��ʵ��һ��ȡ5öȥ��ȥ��Ľྻ�������ֱ�װ���±����Թ��У�����ʵ�顣
ʵ��ͼʾ |
|
|
|
|
|
����ʱ�� | 8min | �ϳ�ʱ�䲻���� | 5min | 3min | 1min |
��1��ͨ������ʵ���֪���¶�Խ�ߣ�������������Խ_____��������족������_____���ϡ���ᡱ����ˮ����ϡ��ˮ�����������������ʽϿ졣
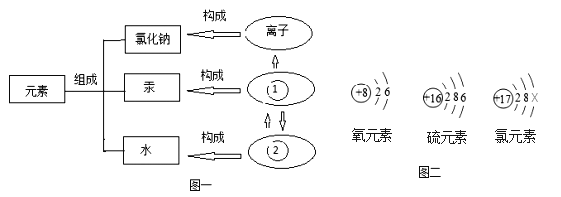
��ʵ�����Ϊ̽������ijɷ֣���ͼ1��ʾװ�ã��г�������ʡ�ԣ�����ʵ�飨ÿ����Ӧ�����վ���ȫ����

���������ϣ���ˮ����ͭ��ˮ���ɰ�ɫ��Ϊ��ɫ����ʯ�Ҽ�����ˮҲ������CO2��
������ʵ�飩
��2��ָ����ͼ1��ʾʵ��װ�õ�һ������ȱ��________��
��3������ǰ����ͨ��COһ��ʱ�䣬Ŀ����_____________��
��4����������Ʒ���ȣ���ˮ����ͭ�������ɴ���֪������һ������______Ԫ�أ��Ӷ��ƶϳ������е�______�μ��������ⷴӦ��
��ʵ���¼��
������Ʒ������/g | װ��B������/g | װ��C������/g | |
��Ӧǰ | 23.2 | 232.4 | 198.2 |
��Ӧ�� | / | 239.6 | 211.4 |
��ʵ���¼��
��5�������ϱ����ݿ���ȷ������ijɷ֡�����FexOynH20��ʾ�� n=_________��
��ȱ��װ��D����x��y��ֵ��________���ƫ����ƫС������Ӱ�족����
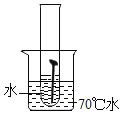
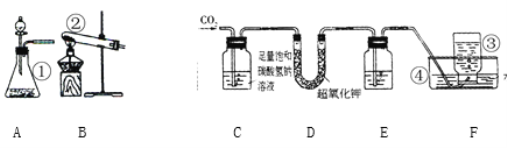
��6����ʹ����ͼ2��ʾװ��̽��þ���ڿ�������ʴ����������֪þ������IJ���Ϊ��ʽ̼��þ[Mg3��OH��2��CO3��2]�����Թ��ڿ�ѡ�õ������У�
��O2 ��ˮ ��CO2 ��O2��CO2 ��CO2��ˮ ��O2��ˮ ��O2��CO2��ˮ
������Ҫѡ��_______������ţ����Ա�ʵ�飬���ܴﵽ̽��þ����ʴ��������
��7��д��þ���ڿ�������ʴ�Ļ�ѧ����ʽ________��
���𰸡��� ϡ���� û�н���β������ �ž�װ���ڵĿ�������ֹ������ը �� ˮ 4 ƫС �ܢݢޢ� 6Mg+3O2+4CO2+2H2O=2Mg3��OH��2��CO3��2
��������
��1���Ա�ʵ��1��ʵ��4��ʵ��3��ʵ��5��֪���¶�Խ��������������Խ�죻�Ա�ʵ��1��2��3��֪��������ϡ��������ʴ�Ͽ죻
��2��β���к����ж���һ����̼���壬��װ����û�н��䴦����
��3��һ����̼���п�ȼ�ԣ�����ʱ���Ȼ��ȼ������ը��������ͨ��COһ��ʱ�䣬�ž�װ���ڵĿ�����
��4����ˮ����ͭ������ˮ����������������Ʒ���ȣ���Ʒ��ڣ���ˮ����ͭ��������˵���˷�Ӧ����ˮ���ɣ�˵�������к�����Ԫ�أ�Ҳ��������ʴ�Ĺ�������ˮ���룻
��5��Ӳ�ʲ������ڷ�����Ӧ�Ļ�ѧ����ʽΪ��FexOynH2O+yCO ![]() xFe+yCO2+nH2O������ʵ�����ݿ�֪����Ӧ������ˮ������=��239.6g-232.4g��=7.2g�����ɶ�����̼������=��211.4g-198.2g��=13.2g��
xFe+yCO2+nH2O������ʵ�����ݿ�֪����Ӧ������ˮ������=��239.6g-232.4g��=7.2g�����ɶ�����̼������=��211.4g-198.2g��=13.2g��
�裺�μӷ�Ӧ��CO������Ϊw����
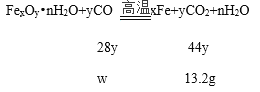
![]() w=8.4g
w=8.4g
�������غ㶨�ɿ�֪��������������Ϊ��23.2g+8.4g-13.2g-7.2g=11.2g�����ڷ�Ӧǰ��Ԫ�ص��������������䣬����23.2gFexOynH2O�к���Ԫ�ص�����Ϊ11.2g�����С�H2O����������=7.2g�������µ���Ԫ������Ϊ��23.2g-11.2g-7.2g=4.8g������![]() ����FexOynH2O�Ļ�ѧʽΪFe2O34H2O��Dװ���еļ�ʯ�Ҽ�������ˮҲ������CO2����ֹ�˿����ж�����̼��ˮ��װ��C���ն��������ƫ����û��װ��D����װ��C��Ϊ�����˿����е�ˮ�����Ͷ�����̼������ƫ���ɴ˼�����Ķ�����̼����ƫ������Ԫ�ص�����ƫ������x��y��ֵ��ƫС��
����FexOynH2O�Ļ�ѧʽΪFe2O34H2O��Dװ���еļ�ʯ�Ҽ�������ˮҲ������CO2����ֹ�˿����ж�����̼��ˮ��װ��C���ն��������ƫ����û��װ��D����װ��C��Ϊ�����˿����е�ˮ�����Ͷ�����̼������ƫ���ɴ˼�����Ķ�����̼����ƫ������Ԫ�ص�����ƫ������x��y��ֵ��ƫС��
��6��ͨ��ʵ��̽��þ���ڿ�������ʴ��������Ӧ��ѡ����������ʵ�飺��O2��CO2����CO2��ˮ����O2��ˮ����O2��CO2��ˮ����ʵ����жԱȣ�����ܢݢޢߣ�
��7����þ���ڿ�������ʴ�����ɼ�ʽ̼��þ[Mg3(OH)2(CO3)2]�����Ʋ⣬��þ��������ˮ�Ͷ�����̼��Ӧ�����˼�ʽ̼��þ����Ӧ�Ļ�ѧ����ʽ��6Mg+3O2+4CO2+2H2O=2Mg3(OH)2(CO3)2��