��Ŀ����
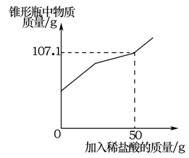
��9�֣���Ϊ�ⶨij��ʯ��ʯ��̼��Ƶ�������������ȤС��ͬѧȡһ��������ʯ��ʯ���ձ��У����ձ��������Ũ�����ᣬ��Ӧ���̲��ʣ�������������������������ϵ��ͼ��ʾ��ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ������ش��������⣺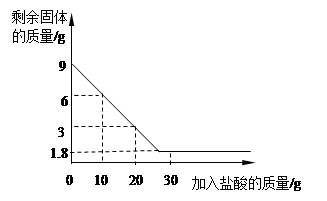
��1��ʯ��ʯ���������ʵ������� g��
��2��ͨ�������������������������������
��3����ȤС��ͬѧ�òⶨ�������������������ʯ��ʯ��̼��Ƶ������������������ȷ��ͨ������õ���̼��Ƶ�����������ʵ����ֵƫ����ԭ������ǣ� ��
��1��1.8 ��2��21.9%��3��������̼�����к��н�Ũ����ӷ����Ȼ�������ʹ��������ʵ�ʲ���������̼�������ɶ�����̼�����̼�������ƫ��
�������������ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ�����Թ�����ٵ�������Ϊ̼��Ƶ�������9-1.8=7.2g�����ʵ�������������ʣ�������Ϊ1.8g����2������������������ʱ��Ҫע�⣬Ӧ���÷�Ӧ����������������㣬���Կ�ѡ��10g����20g����10g����������HCl������Ϊx (Ҳ����20g������㣬������������Ҳ�÷�)CaCO3+2HCl=CaCl2+H2O+CO2��
100 73
9g-6g X 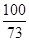 =
=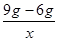
x=2.19g
���������ʵ���������=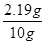 ��100%=21.9%
��100%=21.9%
��3��������̼�����к��н�Ũ����ӷ����Ȼ�������ʹ��������ʵ�ʲ���������̼�������ɶ�����̼�����̼�������ƫ��
���㣺���ݻ�ѧ����ʽ���м���

����ʯ��ʯ���Ƶ������ƣ�����ʯ��ʯ�е����ʲ����뷴Ӧ���Ҳ����ơ�̼Ԫ�أ���������պ�ʣ������и�Ԫ����̼Ԫ�ص�������Ϊ20��3�����ѷֽ��̼���ռԭ̼��Ƶ���������Ϊ�� ��
| A��40% | B��60% | C��30% | D��50% |
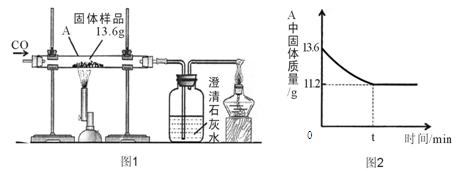
��7�֣�ѧ��������Ժ���˼ͬѧͨ��ʵ��̽��п����������������������������Һ�ķ�Ӧ��ʵ��������������£�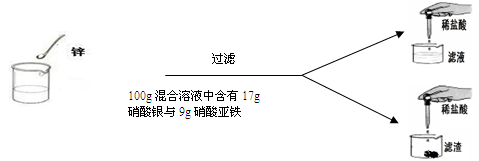
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������������Ļ����Һ | 100g | 100g | 100g | 100g |
| � | 2g | 3.25g | m | 9.75g |
| ����Һ�м���ϡ������ʵ������ | ������ɫ���� | ���������� | ���������� | ���������� |
| �������м���100 gϡ������ʵ������ | ���������� | ���������� | �������ݣ���Һ��Ϊdz��ɫ | �������������ͬ |
��2�����ݵ�һ�ε�ʵ����������Ϊ�˴���Һ�е������� ��
��3��������֪�����г����ڶ���ʵ�����û���������������(x)�ı���ʽ ��
��4����������ʵ��п����������Һǡ����ȫ��Ӧ����m������Ϊ__________________��
��5����������η�Ӧ�����Һ�м���һ��������ˮ�����ò�������Һ�����ʵ���������Ϊ10%�������ˮ������Ϊ__________________��
��6������������������Ϊ36.5����Ũ�������Ƶ��Ĵ�ʵ����������ǡ����ȫ��Ӧ�����ϡ���ᣬ����ҪŨ������ˮ��������Ϊ_______________��