��Ŀ����
ζ���dz��õĵ�ζƷ��ijζ���ɹȰ����ƣ���ѧʽΪC5H8NO4Na�����������Ȼ�����ɣ�Ϊ�˲ⶨ��ζ���йȰ����Ƶĺ�������������ʵ�飺ȡ��ζ����Ʒ5.50�ˣ���������ˮ�У�������������������Һ����Ӧ�Ļ�ѧ����ʽΪ��NaCI+AgNO3==AgCl��+NaNO3�����Ȱ���������������Һ����Ӧ����ַ�Ӧ����ˡ�ϴ�ӡ���ɡ��������õ�����1.35�ˡ����㣺
��1����ζ���йȰ����Ƶ���������������������ȷ��1����
��2��ʳ�ø�ζ��3.60�ˣ���Ϊ�����ṩ���ٿ���Ԫ��?����������ȷ��0.01�ˣ�
��1����ζ����Ʒ�к��Ȼ��Ƶ�����Ϊ![]() ��
��
NaCl+AgNO3==AgCl��+NaNO3
58.5 143.5
![]() 1.35g
1.35g
58.5��143.5=![]() ��1.359
��1.359
��ã�![]() =0��550g
=0��550g
���ζ���йȰ����Ƶ���������Ϊ����5.50g��0.550g����5.50g��100��=90��
��2��3.6gζ����Ʒ�к��йȰ����Ƶ�����Ϊ��3.6g��90��=3.24g
��ʳ��3��6gζ������Ԫ������Ϊ��3.24g��23��169��100��+��3.6g��3.24g����23��58��5��100��=0.58g
�𣮣��ԣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
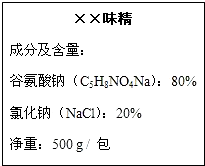 ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�
ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�