��Ŀ����
ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na��������ˮ����AgNO3����Ӧ�����������NaCl�������ɷֲ����ǣ�����ش��������⣺
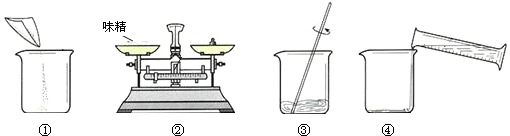
��1����ȡ5.0gζ�����Ƴ�50g��Һ����ȡ����ˮ����Ͳ�����
A.10mL B.50mL C.100mL
��2����ͼ�����ƹ��̣���ȷ�IJ���˳��Ϊ

��3��Ϊ�ⶨζ����NaCl��������������������ʵ�飺
���������Ƶ�50g��Һ�м��������AgNO3��Һ��ַ�Ӧ��
�ڹ��˺�ϴ�ӡ��������AgCl���壮
����������������ȷ������£�����������Һ�����У����ӿ̶���ȡ����ˮ������������Һ�ĹȰ�������������
��1����ȡ5.0gζ�����Ƴ�50g��Һ����ȡ����ˮ����Ͳ�����
B
B
��������ĸ��A.10mL B.50mL C.100mL
��2����ͼ�����ƹ��̣���ȷ�IJ���˳��Ϊ
�ڢ٢ܢ�
�ڢ٢ܢ�
��������ţ�
��3��Ϊ�ⶨζ����NaCl��������������������ʵ�飺
���������Ƶ�50g��Һ�м��������AgNO3��Һ��ַ�Ӧ��
�ڹ��˺�ϴ�ӡ��������AgCl���壮
����������������ȷ������£�����������Һ�����У����ӿ̶���ȡ����ˮ������������Һ�ĹȰ�������������
ƫС
ƫС
���ƫ����ƫС������Ӱ�족�������õ���Ʒ���Ȼ��Ƶ�����������Ӱ��
��Ӱ��
���ƫ����ƫС������Ӱ�족������������1��ѡȡ��Ͳ��ѭ��һ���������ԭ��
��2��������Һ�IJ����ǣ����㡢�������ܽ⣻
��3�������Ӷ���ʱ�������������С��ʵ��Һ������������ζ����Һֻ��Ϊ������Һ�ڽ��з�Ӧ�����ݴ�������Һ���Ȼ��Ƶ����������أ�
��2��������Һ�IJ����ǣ����㡢�������ܽ⣻
��3�������Ӷ���ʱ�������������С��ʵ��Һ������������ζ����Һֻ��Ϊ������Һ�ڽ��з�Ӧ�����ݴ�������Һ���Ȼ��Ƶ����������أ�
����⣺��1����ȡ5.0gζ�����Ƴ�50g��Һ����Ҫ����ˮ��������45g�������45mL��Ϊ�˼�С��Ҫһ����ȡ��ȡ��Ͳ���������ȡ����ˮ�������ӽ�����Ͳ--50mL����Ͳ��
��2������������Һ�IJ����֪���ȳ������壬�����ձ�������ȡˮ�����ձ��������ܽ⣻
��3��������ʵ����ȡˮ����������Ӷ���������ʱ����Ҫˮ���������ˣ�ʹ�����Ƶ���Һ��ˮ��ƫ�����������ƫС���ڲⶨ���̺�����ݴ����У�����Ҫʹ��������ζ����Һ��������������ˣ�����Ӱ�����յIJⶨ�����
�ʴ�Ϊ��
��1��B��
��2���ڢ٢ܢۣ�
��3��ƫС����Ӱ�죮
��2������������Һ�IJ����֪���ȳ������壬�����ձ�������ȡˮ�����ձ��������ܽ⣻
��3��������ʵ����ȡˮ����������Ӷ���������ʱ����Ҫˮ���������ˣ�ʹ�����Ƶ���Һ��ˮ��ƫ�����������ƫС���ڲⶨ���̺�����ݴ����У�����Ҫʹ��������ζ����Һ��������������ˣ�����Ӱ�����յIJⶨ�����
�ʴ�Ϊ��
��1��B��
��2���ڢ٢ܢۣ�
��3��ƫС����Ӱ�죮
������������������Һ�IJ��衢��Ͳѡȡ�����������˽����֪ʶ�������Ӧ���ǽ�����Ļ����ؼ�����������Ͳ��ʹ�ö���������Ӱ�������Ҫϸ�ķ������

��ϰ��ϵ�д�
�����Ŀ
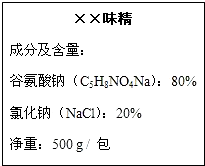 ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�
ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�